Research Article
Baseline Bone Mineral Density Assessment and Osteoporosis Risk in Early Breast Cancer Patients Undergoing Aromatase Inhibitor Therapy
- Shahid Gilani
Corresponding author: Shahid Gilani, University Hospitals of North Midlands, Staffordshire, United Kingdom.
Volume: 2
Issue: 3
Article Information
Article Type : Research Article
Citation : Shahid Gilani, Syed Hammad Hassan Tirmazy, Muhammad Farooq Latif, Naima Ikram. Baseline Bone Mineral Density Assessment and Osteoporosis Risk in Early Breast Cancer Patients Undergoing Aromatase Inhibitor Therapy. Journal of Medical and Clinical Case Reports 2(3). https://doi.org/10.61615/JMCCR/2025/JULY027140729
Copyright: © 2025 Shahid Gilani. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.61615/JMCCR/2025/JULY027140729
Publication History
Received Date
12 Jul ,2025
Accepted Date
23 Jul ,2025
Published Date
29 Jul ,2025
Abstract
Background
Breast cancer is a heterogeneous disease characterised by diverse molecular subtypes and receptor profiles, including oestrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) status. Treatment strategies for early breast cancer rely heavily on surgical intervention followed by adjuvant therapies tailored to these receptor subtypes. ER/PR-positive tumours are commonly managed with hormone therapies such as tamoxifen or an aromatase inhibitor (AI), while HER2-positive cancers may benefit from targeted therapies like trastuzumab. However, aromatase inhibitors, which are particularly effective in postmenopausal ER/PR-positive breast cancer, significantly reduce circulating oestrogen levels, consequently accelerating bone mineral density (BMD) loss and elevating the risk of osteopenia, osteoporosis, and fragility fractures. This is of particular concern given the role of oestrogen in inhibiting osteoclast activity and preserving skeletal integrity.
Methods
In this retrospective study, we evaluated baseline BMD in women with early breast cancer who were prescribed aromatase inhibitors. Between January and June 2015, 289 women were followed over five years.
Results
Of these, 219 had ER/PR-positive disease, with 93% of postmenopausal patients initiated on AI. Baseline DEXA scans were performed in 102 patients (75%), revealing that 20% had osteoporosis (T-score < -2.5) and 54% had osteopenia (T-score between -1 and -2.5), indicating that 74% had compromised bone health at therapy initiation. Furthermore, 60% of these patients had additional osteoporosis risk factors, including low BMI, smoking, corticosteroid use, and comorbid conditions such as diabetes and COPD. Bisphosphonates were prescribed to patients diagnosed with osteoporosis, and 52% underwent follow-up DEXA scans during AI therapy. Importantly, the presence of low BMD or bisphosphonate therapy showed no statistically significant association with progression-free survival (PFS) within the five-year follow-up period.
Conclusion
This study highlights that a significant proportion of early breast cancer patients exhibit compromised bone health at the onset of AI therapy, underscoring the necessity for routine BMD assessments and proactive management strategies.
Highlights
High prevalence (74%) of low BMD in early breast cancer patients initiating AI therapy.
Baseline DEXA scans are crucial for identifying patients at risk of osteoporosis.
Co-morbidities such as diabetes and rheumatoid arthritis contribute to increased osteoporosis risk.
Osteoporosis or its treatment did not impact progression-free survival over a 5-year follow-up.
Regular bone health monitoring and lifestyle modifications are essential components of patient management.
►Baseline Bone Mineral Density Assessment and Osteoporosis Risk in Early Breast Cancer Patients Undergoing Aromatase Inhibitor Therapy
Shahid Gilani1*, Syed Hammad Hassan Tirmazy2, Muhammad Farooq Latif3, Naima Ikram4
1,4University Hospitals of North Midlands, Staffordshire, United Kingdom.
2,3Dubai Hospital, Dubai Academic Health Corporation, UAE. University Hospital of North Midlands, Staffordshire, United Kingdom.
Introduction
Breast cancer encompasses diverse diseases, exhibiting distinct clinicopathological and genomic characteristics. The primary treatment for early breast cancer is surgical resection. Further treatment approach depends on the presence of molecular subtypes and hormone receptor status, such as oestrogen receptors (ER) and/or progesterone receptors (PR) and human epidermal growth factor receptor 2 (HER2). ER/PR and HER2 receptor-positive breast cancer refers to breast cancer cells that have specific receptors on their surface that influence their growth. Understanding these receptors helps determine treatment options.
ER/PR positive breast cancers are typically treated with hormone therapy (like tamoxifen or aromatase inhibitors), which blocks hormones from fuelling cancer growth. Some breast cancers are ER-positive, PR-positive, and HER2-positive. These cases respond to both hormone therapy and HER2-targeted treatments, giving patients multiple treatment options, whereas some breast cancers lack all three receptors, known as triple-negative breast cancer. [1]. For premenopausal women, hormonal therapies consist of selective oestrogen receptor modulators (SERMS) or ovarian suppression/ablation through surgical or medical means, enabling them to receive AI [2]. Before and after menopause, oestradiol (E2) and oestrone (E1) are the primary oestrogens in the bloodstream. During the premenopausal phase, the ovaries maintain circulating oestrogen levels. However, after menopause, as ovarian oestrogen synthesis decreases, oestrogen is produced in peripheral tissues through the aromatisation of androgens [3]. Oestrogen is vital in inhibiting osteoclasts, the cells responsible for breaking down bone tissue. Therefore, a deficiency of oestrogen after menopause can lead to loss of bone mineral density (BMD), posing an increased risk of fractures [4].
Osteoporosis is a significant concern for breast cancer patients taking aromatase inhibitors (AI) such as anastrozole, letrozole, and exemestane. These drugs lower oestrogen levels, which is crucial for postmenopausal women with hormone-receptor-positive breast cancer, but oestrogen is also essential for maintaining bone density. The resulting oestrogen deficiency accelerates bone loss, increasing the risk of osteopenia, osteoporosis, and fractures [5]. AI decreases oestrogen, which generally suppresses bone resorption, leading to increased bone breakdown. AI users experience a 2-3% loss of Bone Mineral Density (BMD) per year, significantly higher than the natural postmenopausal loss. The risk of fractures (hip, spine, and wrist) is increased, with some studies showing up to a 50% higher fracture rate compared to those not taking AI [6].
Independent risk factors for osteoporosis in AI users are low BMD (osteopenia or osteoporosis) at the outset, previous history of fractures, older women, family history of osteoporosis, low body weight (BMI < 20), smoking and excessive alcohol intake, vitamin D and calcium deficiency, lack of weight-bearing exercise and use of corticosteroids or other bone-affecting medications [8]. Patients with breast cancer, especially those on AI, should undergo regular bone health monitoring. The primary tool for this is Dual-Energy X-ray Absorptiometry (DEXA) scanning, along with biochemical markers of bone turnover when necessary [8].
Prevention and management strategies include bone density monitoring with baseline and regular DEXA scans (every 1-2 years) to track BMD loss, calcium and vitamin D supplementation (Calcium: 1,000-1,500 mg/day and Vitamin D: 800-2,000 IU/day), weight bearing and resistance exercises (strength training and walking can help maintain bone strength). Lifestyle Modifications help to improve bone health, including quitting smoking, reducing alcohol intake, and maintaining a healthy diet rich in dairy, leafy greens, and fish. Pharmacological bone-protective Medications include bisphosphonates (e.g., alendronate, zoledronic acid) to reduce fracture risk. Denosumab, a monoclonal antibody that inhibits bone resorption, is an option for those intolerant to bisphosphonates. Raloxifene, a selective oestrogen receptor modulator (SERM), is sometimes used in specific cases. If bone loss is severe, switching AI to tamoxifen may be considered [5, 6, 7].
Given the potential skeletal complications associated with AI therapy, it is imperative to assess baseline bone mineral density (BMD) and implement strategies to monitor and manage bone health in breast cancer patients. We conducted a retrospective study to evaluate baseline BMD assessment in patients with breast cancer to look at the incidence of osteopenia and osteoporosis. This study aims to assess the baseline BMD and the prevalence of bone density loss in early breast cancer patients undergoing AI therapy, emphasizing the importance of bone health monitoring in this population. The study was previously presented in an abstract at the ESMO Breast Cancer conference, which took place in Berlin, Germany, on 3-5 May 2022.
Methods
Study Design and Population
A retrospective cohort study was conducted involving 289 women diagnosed with early breast cancer between January and June 2015. Patients were followed for a period of 5 years. Data was collected regarding patients’ demographics, menopausal status, co-morbidities, histological subtypes, disease stage, use of adjuvant endocrine treatments, DEXA scan findings at the baseline, and follow-up DEXA scan results.
Data Collection
Clinicopathological data, including hormone receptor status (ER/PR, HER2), menopausal status, and adjuvant therapy details, were collected. BMD was assessed using Dual-Energy X-ray Absorptiometry (DEXA) scans at baseline and during follow-up.
BMD Classification based on World Health Organization criteria
- Normal: T-score ≥ -1.0
- Osteopenia: T-score between -1.0 and -2.5
- Osteoporosis: T-score ≤ -2.5
Statistical Analysis
Descriptive statistics were used to summarize patient characteristics and BMD findings. Progression-free survival (PFS) was analyzed concerning baseline BMD status and bisphosphonate therapy. Univariate and multivariate analyses performed. Kaplan-Meier survival curves were created for patients with or without osteoporosis at baseline. Statistical analysis was performed by using SPSS v. 29
Results
Patient Characteristics
Among the 289 patients, 219 (75.8%) were ER/PR-positive, 40 (13.8%) were HER2-positive, and 15 (5.2%) were triple-negative. Of the ER/PR-positive patients, 140 (64%) were postmenopausal, and 79 (36%) were pre- or peri-menopausal.
Baseline BMD Assessment
Baseline DEXA scans were performed in 102 patients receiving AI therapy. The distribution of BMD categories is presented in Table 1A and Table 1B.
Table 1A: Demographics And Clinicopathological Data Of All Females With A Mean Age: 76 (SD=11.7).
HR+ (hormone positive), HER2+ (human epithelial receptor-2 positive), TNBC (triple negative breast cancer), DCIS (ductal carcinoma in situ), LCIS (lobular carcinoma in situ), DEXA (dual-energy X-ray absorptiometry), AI (aromatase inhibitor). *A T-score results from a bone density test (DXA scan) that compares bone density to the average bone density of a healthy young adult of the same sex. A T-score of -2.5 or lower indicates osteoporosis, while a T-score between -1 and -2.5 suggests osteopenia (low bone density), and a T-score of -1 or higher is considered normal.
|
Total patients with breast cancer (289) |
Invasive cancer 87% (252) |
HR+ 80% (219) |
HER2+ 18% (40) |
TNBC 6% (15) |
DCIS/LCIS 13% (37) |
|
Patients |
On AI 97% (102) |
On Tamoxifen 2% (3) |
No endocrine Rx (11) |
First DEXA 75% (76) |
2nd DEXA 40% (34) |
|
Breast Cancer Treatment |
WLE SLNB and radiotherapy 48% |
Primary Hormone Therapy 31% |
Mastectomy 19 % |
Chemotherapy 27% |
|
|
Risk factors of bone loss |
Co-morbidities (DM, COPD, RA) 60%
|
Ex-Smoker 52% |
Smoker 8% |
H/O fractures 8% |
|
|
DEXA results |
|
*Normal (T score ≥ -1) |
*Osteopenia (T <-1 > -2.5) |
*Osteoporosis (T score ≤ -2.5) |
|
|
First scan |
27 |
57 |
21 |
|
|
|
2nd scan |
8 |
28 |
6 |
|
Table 1B: The Distribution of BMD Categories Is Presented.
|
Table 1B. Baseline BMD Categories in Patients Undergoing AI Therapy |
||||||||||||
|
Risk Factors for Low BMD
Approximately 60% of patients with low BMD had at least one additional risk factor for osteoporosis, including comorbidities such as diabetes mellitus, chronic obstructive pulmonary disease (COPD), rheumatoid arthritis (RA), history of low-impact fractures, smoking, and low body mass index (BMI < 20).
Management and Follow-Up
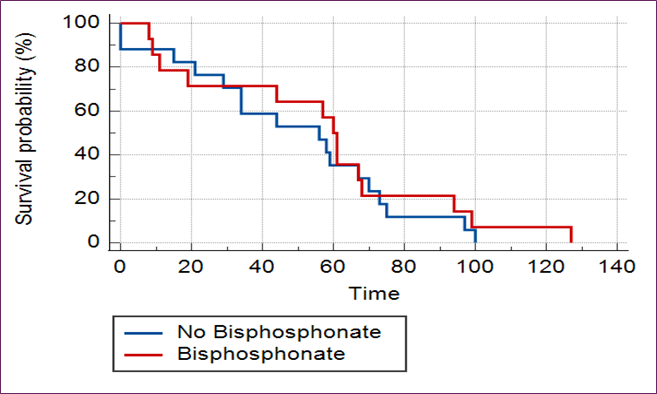
Patients diagnosed with osteoporosis received bisphosphonate therapy. During AI therapy, 52% of patients had a follow-up DEXA scan. No significant impact on progression-free survival was observed in patients with osteoporosis or those receiving bisphosphonate therapy over the five-year follow-up period [Figs 1A and 1B].
Fig 1A: No Difference Found In Progression-Free Survival (PFS) In Patients with Or Without Osteoporosis
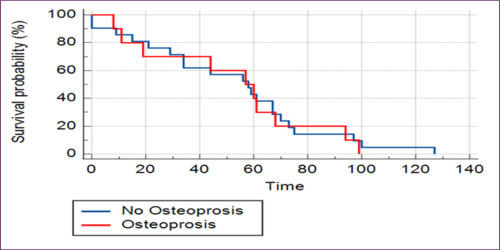
Fig 1B: No Difference In Progression-Free Survival (PFS) Found In Patients with Osteoporosis Treated With Bisphosphonates In Short-Term Follow Up For 5 Years.
Discussion
Our findings highlight a critical gap in bone health management among breast cancer patients initiating AIs. Despite guideline recommendations, only 75% had baseline BMD assessment, and just over half had repeat scans. Early identification of osteopenia or osteoporosis allows timely initiation of bone-protective interventions, including calcium/vitamin D supplementation, exercise, lifestyle changes, and pharmacologic treatment. This aligns with global data: studies show up to 60% prevalence of bone loss in AI candidates. Guidelines from ASCO and ESMO advocate regular DEXA scanning, yet adherence is poor, with some cohorts showing less than 50% baseline testing. Our study underscores the need for quality improvement measures, such as electronic reminders or embedded care pathways, to ensure appropriate screening.
Osteoporosis is a significant global health concern, affecting an estimated 200 million women worldwide. The prevalence of osteoporosis increases with age, with approximately 3.3% of women aged 45–49 being affected, rising to 6.4% for those aged 50–54, 13.5% for those aged 55–59, and reaching a substantial 50.3% in the highest age group of 85 years [1]. Osteoporosis poses a significant health risk for women with breast cancer receiving AI. Healthy postmenopausal women may experience a 1% annual loss of BMD. Whereas women with breast cancer have 2-3 times more bone mineral loss [2], increasing their fracture risk [2,3]. This leads to reduced functionality and poor health-related quality of life (HRQOL), leading to higher morbidity [5].
Postmenopausal women on aromatase inhibitors (AIs), premenopausal women on ovarian suppression therapy, and patients with pre-existing osteopenia/osteoporosis or with risk factors for osteoporosis (e.g., low BMI, smoking, corticosteroid use, history of low-impact fractures, diabetes mellitus, COPD, and rheumatoid arthritis) need regular bone density monitoring [8]. All patients starting AI should have a baseline DEXA scan to assess BMD. A T-score is the result of a bone density test (DXA scan) that compares your bone density to the average bone density of a healthy young adult of the same sex. A T-score of -2.5 or lower indicates osteoporosis, while a T-score between -1 and -2.5 suggests osteopenia (low bone density), and a T-score of -1 or higher is considered normal. If osteoporosis is found at the outset, bone-protective therapy (e.g., bisphosphonates or denosumab) should be considered [7]. For low risk (Normal BMD, T-score ≥ -1.0), repeat the DEXA scan every 2 years, for moderate risk (Osteopenia, T-score between -1.0 and -2.5), repeat every 1–2 years; and for high risk (Osteoporosis, T-score ≤ -2.5 or history of fractures), repeat annually and start bone-protective treatment [6], [Table 1].
Sometimes, additional bone health assessments are required by assessing serum 25- hydroxyvitamin D, ensuring levels are >30 ng/ml (to be supplemented if deficient), corrected calcium levels, and renal function (especially when considering bisphosphonates). Bone turnover markers are optional but useful in high-risk patients by measuring CTX (C-terminal telopeptide), a marker of bone resorption, and P1NP (Procollagen Type 1 N-terminal Propeptide), a marker of bone formation [7].
Choosing the right treatment is important, including lifestyle and nutritional interventions. Pharmacologic treatments based on BMD and fracture risk include bisphosphonates (e.g., alendronate, zoledronic acid), and are the first line for most breast cancer patients with osteoporosis. Denosumab remains an effective alternative when bisphosphonates are contraindicated [8].
The American Society of Clinical Oncology (ASCO), the European Society of Medical Oncology (ESMO), and other cancer educational associations published guidelines for managing bone health in women with BC and recommending BMD screening followed by annual assessments [6, 7, 8]. There are several risk factors for osteoporosis, including older age (>65 years), family history of hip fracture, history of nontraumatic fracture, low weight of < 70 kg, postmenopausal patients receiving AI, and patients with BC therapy-associated menopause [9]. Several studies have reported poor adherence to BMD assessment in patients with breast cancer treated with AI. In a retrospective cohort study by Spangler et al, 56% of women had no BMD tests performed within 14 months of starting AI [10]. A quality improvement clinical audit showed that only half of the eligible patients were assessed at baseline [11]. In our study, 75% of postmenopausal patients on AI underwent baseline BMD assessment. We found that 20% of patients in the range of osteoporosis with a T score lower than -2.5, 54% had BMD between -1 to -2.5, consistent with osteopenia, and 26% of them were found to have a normal T score above -1. A study conducted in Spain also revealed a high prevalence of bone mass loss (62.2%) in patients before initiating AI treatment, with 16.5% having osteoporosis and 45.7% exhibiting osteopenia [12]. We found that the second DEXA scan was done in about half of them (52%) during treatment. A large retrospective study using the SEER-Medicare database showed that among patients who underwent a baseline DEXA scan and continued AI for 3 years, 28.0% had a repeat DEXA scan within 2 years and 65.9% within 3 years [13].
Risk factors for osteoporosis are well known and include lifestyle factors, diet, low weight, age, and co-morbidities like DM, COPD, and RA [14]. In our study, 60% of women had risk factors for osteoporosis, and 40% of patients were treated with bisphosphonates. Several studies have reported mixed results on breast cancer outcomes treated with adjuvant bisphosphonates. However, a meta-analysis conducted by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) showed that the addition of adjuvant bisphosphonates was associated with a significant reduction in the 10-year risk of bone recurrence (7.8 per cent versus 9.0 per cent). Although the authors observed reductions at 10 years for distant recurrence (20.4 per cent versus 21.8 per cent) and breast cancer mortality (16.6 per cent versus 18.4 per cent); these differences were of only borderline statistical significance [15]. Our study has shown no PFS in patients with osteoporosis or osteopenia or their treatment with bisphosphonates in short-term follow-up for five years [Fig 1A and Fig 1B].
The future of bone mineral density (BMD) assessment is evolving with advancements in biomarkers, imaging technologies, and precision therapies. Traditional markers like serum calcium, vitamin D, and bone turnover markers (e.g., P1NP, CTX) are being supplemented with novel biomarkers such as sclerostin & dickkopf-1 (Dkk-1), which are the regulators of Wnt signalling, a key pathway in bone formation. MicroRNAs (miRNAs) are emerging as potential indicators of bone metabolism. Similarly, exosomal biomarkers are small extracellular vesicles carrying bone-specific proteins or RNA. Combining proteomics, metabolomics, and genomics may form a comprehensive bone health assessment. Future diagnostics may enable rapid, non-invasive biomarker analysis via blood, urine, or saliva [17].
Advanced imaging and scanning techniques like high-resolution imaging with HR-pQCT (High-Resolution Peripheral Quantitative Computed Tomography) provide ultra-detailed images of bone microarchitecture. MRI-based bone imaging uses quantitative MRI for bone quality assessment without radiation exposure. Artificial Intelligence (AI) -driven dual-energy X-ray absorptiometry (DXA) and CT scan analysis can enhance accuracy. Machine learning models to predict fracture risk from standard X-ray images may enhance rapid diagnosis. Hybrid approaches like PET/CT and SPECT/CT integrate metabolic and structural data. Ultrasound-based techniques (e.g., pulse-echo ultrasound) are expected to promote portable and radiation-free assessment [18].
The integration of biomarkers, AI-driven imaging, and precision therapies will create a comprehensive bone health ecosystem. The development of wearable and non-invasive devices for continuous bone health monitoring would be user-friendly. Advances in pharmacogenomics to match patients with the most effective osteoporosis treatments would lead to precision therapies for osteoporosis and bone disorders [17]. Looking forward, precision diagnostics may revolutionize bone health assessment. Emerging biomarkers (e.g., sclerostin, Dkk-1, miRNAs), high-resolution imaging (HR-pQCT), and AI-driven risk prediction tools promise individualized risk stratification. These advancements, coupled with patient-friendly non-invasive monitoring tools, could transform osteoporosis care in oncology settings.
Limitations of our study include the retrospective single-centre design and modest follow-up duration. Nonetheless, our findings reinforce the importance of integrating bone health strategies in oncology, especially for patients at high fracture risk.
Highlights
- Baseline bone loss is common: 74% of postmenopausal women with early breast cancer initiating aromatase inhibitor therapy had either osteopenia or osteoporosis.
- High-risk profile: 60% of patients with low BMD also had additional osteoporosis risk factors such as low BMI, smoking, corticosteroid use, and chronic co-morbidities.
- Underutilization of follow-up scans: Only 52% of patients with compromised BMD underwent follow-up DEXA scans during aromatase inhibitor therapy.
- Clinical outcomes: No significant association was found between low BMD or bisphosphonate therapy and five-year progression-free survival.
- Call for routine screening: Early and regular DEXA scans are critical to identify patients who may benefit from bone-protective strategies.
- Future directions: Emerging tools like AI-driven imaging, bone turnover biomarkers, and pharmacogenomics may enhance personalized bone health management in breast cancer care.
CRediT Authorship Contribution Statement
SG: Corresponding author played a substantial role contribution to the acquisition, interpretation, drafting, revising intellectual content, final drafting, and approval. SHT: Initial drafting. MFL: Initial drafting. NI: Acquisition of data.
Declaration Of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Approval
Clinical audit department at University Hospital of North Midlands, UK: Reg. Number CA15021.
Funding Declaration
The authors declare no funding from any source.
Ethics Statement
This retrospective study was conducted after approval and registration from the clinical audit department at the University Hospital of North Midlands, UK. Individual subject consent was not necessary. Human Ethics and Consent to Participate declarations: not applicable.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conclusion
The study reveals a high prevalence of bone density loss in early breast cancer patients at the initiation of AI therapy, with 74% exhibiting osteopenia or osteoporosis. Despite guidelines recommending baseline BMD assessments, only 75% of patients underwent DEXA scans, indicating a gap in clinical practice. The lack of impact of osteoporosis or its treatment on short-term PFS suggests that while bone health is crucial for overall well-being, it may not directly influence cancer progression within a 5-year timeframe. These findings underscore the need for routine BMD monitoring and proactive management of bone health in breast cancer patients, particularly those receiving AIs.
- Eroles P, Bosch A, Alejandro Pérez-Fidalgo J, Lluch A. (2012). Molecular biology in breast cancer: Intrinsic subtypes and signaling pathways. Cancer treatment reviews. 38(6): 698-707.
- Dowsett M, Forbes JF, Bradley R. (2015). Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. The Laincet (British edition). 386(10001): 1341-1352.
- Burger HG. (2006). Physiology and endocrinology of the menopause. Medicine (Abingdon. 1995, UK ed.). 34(1): 27-30.
- Boschitsch EP, Durchschlag E, Dimai HP. (2017). Age-related prevalence of osteoporosis and fragility fractures: Real-world data from an austrian menopause and osteoporosis clinic. Climacteric : the journal of the International Menopause Society. 20(2): 157-163.
- Brown SA, Guise TA. (2007). Cancer-associated bone disease. Current osteoporosis reports. 5(3): 120-127.
- Peyman Hadji. (2009). Aromatase inhibitor-associated bone loss in breast cancer patients is distinct from postmenopausal osteoporosis. Critical reviews in oncology/hematology. 69(1): 73-82.
- Charles L Shapiro, Catherine Van Poznak, Christina Lacchetti, Jeffrey Kirshner, Richard Eastell, Robert Gagel, Sean Smith, Beatrice J Edwards, Elizabeth Frank, Gary H Lyman, Matthew R Smith, Rahul Mhaskar, Tara Henderson, Joan Neuner. (2019). Management of Osteoporosis in Survivors of Adult Cancers With Nonmetastatic Disease: ASCO Clinical Practice Guideline, J Clin Oncol. 37(31): 2916-2946.
- R Coleman, P Hadji, J-J Body, D Santini, E Chow 5, E Terpos, S Oudard, Bruland, P Flamen, A Kurth, C Van Poznak, M Aapro, K Jordan. (2020). Bone health in cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 31(12): 1650-1663.
- HILLNER BE, INGLE JN, BROWN S. (2003). American society of clinical oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. Journal of clinical oncology. 21(21): 4042-4057.
- SPANGLER L, YU O, LOGGERS E, BOUDREAU DM. (2013). Bone mineral density screening among women with a history of breast cancer treated with aromatase inhibitors. Journal of women's health (Larchmont, N.Y. 2002). 22(2): 132-140.
- Zekri J, Farag K. (2016). Assessment of bone health in breast cancer patients starting adjuvant aromatase inhibitors: A quality improvement clinical audit. Journal of Bone Oncology. 5(4): 159-162.
- Gil RHS, Rodríguez EMR, Rodríguez LMR. (2021). Most women with breast cancer about to initiate aromatase inhibitors already have bone loss. Medicina Clínica Práctica. 4(1): 100156.
- Stratton J, Hu X, Soulos PR. (2017). Bone density screening in postmenopausal women with early-stage breast cancer treated with aromatase inhibitors. Journal of Oncology Practice. 13(5): 505-515.
- Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. (2018). A comprehensive overview on osteoporosis and its risk factors. Therapeutics and Clinical Risk Management. 14: 2029-2049.
- Coleman R, Powles T, Paterson A. (2015). Adjuvant bisphosphonate treatment in early breast cancer: Meta-analyses of individual patient data from randomised trials. The Lancet (British edition). 386(10001): 1353-1361.
- S. Gilani, N. Ikram. (2022). Bone Mineral Density screening and monitoring in Early Breast Cancer patients treated with Aromatase Inhibitors. Annals of Oncology. 33(3): 224-231.
- Giovanni Adami, Angelo Fassio, Davide Gatti, Ombretta Viapiana, Camilla Benini, Maria I Danila, Kenneth G Saag, Maurizio Rossini. (2022). Osteoporosis in 10 years time: a glimpse into the future of osteoporosis .Ther Adv Musculoskelet Dis. 14:1759720X221083541.
- Chen-I Hsieh, Kang Zheng, Chihung Lin, Ling Mei, Le Lu, Weijian Li, Fang-Ping Chen, Yirui Wang, Xiaoyun Zhou, Fakai Wang, Guotong Xie, Jing Xiao, Shun Miao & Chang-Fu Kuo. (2021). Automated bone mineral density prediction and fracture risk assessment using plain radiographs via deep learning. Nature communications. 12(1): 5472.
Download Provisional PDF Here
PDF




p (1).png)




.png)




.png)
