Review Article
Oral Manifestations of Cancer and Their Management: A Systematic Review
- Momna Arshad
Corresponding author: Momna Arshad, Master in Public Health, Nottingham Trent University, UK.
Volume: 2
Issue: 1
Article Information
Article Type : Review Article
Citation : Ravibhai M Rami, Momna Arshad. Oral Manifestations of Cancer and Their Management: A Systematic Review. Journal of Dentistry and Oral Health, 2(1). https://doi.org/10.61615/JDOH/2025/NOV027141120
Copyright: © 2025 Momna Arshad. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.61615/JDOH/2025/NOV027141120
Publication History
Received Date
30 Oct ,2025
Accepted Date
15 Nov ,2025
Published Date
20 Nov ,2025
Abstract
Oral manifestations of cancer, including primary malignancies like squamous cell carcinoma presenting as painful ulcers and erythroleukoplakia, treatment-related complications such as mucositis in 80% of radiotherapy patients and dysgeusia in 70% of oral cancer cases, and metastatic lesions from lung or breast primaries manifesting as jaw nodules in 1-3% of oral tumors, significantly impair quality of life, nutrition, and treatment adherence, with pediatric patients experiencing threefold higher acute issues like caries and infections compared to adults. This systematic review, adhering to PRISMA guidelines, synthesizes evidence from 13 high-quality studies on these manifestations, revealing that early detection via tools like toluidine blue (99% sensitivity for dysplasia) and biomarkers (e.g., miR-21) reduces mortality by halting progression from oral potentially malignant disorders (OPMDs) with 0.5-10% transformation rates, while management strategies including oral hygiene protocols decrease mucositis severity by 30-50%, zinc supplements aid partial dysgeusia recovery in 70%, pilocarpine provides xerostomia relief in 50-70%, and antifungals resolve candidiasis in 80-90%, though gaps persist in chronic taste recovery (persistent in 20-30%) and palliative care for dysphagia and pain in 30-45% of terminal cases. Findings also highlight immunotherapy-induced irAEs like lichenoid mucositis in 10-20% of patients, managed with topical steroids, achieving 60-80% resolution, and microbiome shifts post-therapy increasing infection risks, emphasizing multidisciplinary approaches with baseline exams and probiotics for mitigation. Metastatic outcomes show poor 3-year survival (10-20%) with paresthesia as a key indicator, underscoring routine screening in systemic cancer patients. Overall, the review demonstrates that integrated oral care, including preventive cryotherapy and low-level laser therapy, reduces complications and improves survival, but calls for randomized trials on underrepresented groups like IBD patients and advancements in AI-driven diagnostics to address disparities and enhance personalized interventions.
Keywords: Oral cancer, manifestations, management, mucositis, dysgeusia, xerostomia, metastasis
►Oral Manifestations of Cancer and Their Management: A Systematic Review
Ravibhai M Rami1, Momna Arshad2*
1Public Health Dentistry, Ahmedabad Dental College and Hospital, Gujarat University, India.
2*Master in Public Health, Nottingham Trent University, UK.
Introduction
Cancer remains a significant global health challenge, with oral manifestations serving as critical indicators for diagnosis, treatment monitoring, and patient support. Primary oral cancers, predominantly squamous cell carcinomas (OSCC), account for over 90% of oral malignancies and are strongly linked to risk factors such as tobacco, alcohol, and human papillomavirus (HPV) infection, often presenting as persistent ulcers or lumps that impair essential functions like speech and swallowing [3]. These manifestations facilitate early intervention but also underscore the need for comprehensive care to manage disease progression and quality of life impacts. Systemic cancers further complicate the oral landscape through metastatic spread and therapy-induced side effects, necessitating a holistic approach in oncology [4].
Epidemiological data highlight regional variations in oral cancer incidence, with high rates in South Asia due to betel nut use, contrasted with smoking-related cases in Western countries [3]. Metastatic oral involvement, though rare at 1-3%, poses diagnostic challenges as lesions mimic benign conditions, commonly originating from lung, breast, or prostate primaries [18]. Treatment-related toxicities, such as dysgeusia affecting 70% of patients, contribute to malnutrition and reduced treatment adherence, emphasizing the need for targeted management strategies [5].
Oral potentially malignant disorders (OPMDs), such as leukoplakia and erythroplakia, serve as precursors with transformation risks ranging from 0.5% to 10% in high-risk subtypes, exacerbated by chronic inflammation or immunosuppression in conditions like inflammatory bowel disease (IBD) [13]. Low public awareness often leads to delayed presentations, negatively impacting survival rates, which underscores the need for enhanced screening and education initiatives [6].
Emerging therapies, such as immune checkpoint inhibitors, introduce novel oral adverse events, including sicca syndrome and mucositis in 10-20% of cases, requiring specialized assessment and intervention to balance therapeutic benefits and harm [9]. In palliative care, symptoms like candidiasis and dry mouth are prevalent yet often underaddressed, highlighting the critical role of dentists in multidisciplinary teams [19].
Technological advancements, including artificial intelligence (AI) and spectroscopy, offer promise for improving detection and management of oral manifestations, but inconsistencies in research, particularly for pediatric and long-term care, necessitate this systematic review to inform evidence-based practices and identify future research directions.
Methodology
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines to ensure methodological rigor and transparency. The primary aim was to synthesize evidence on the oral manifestations of cancer—encompassing primary, metastatic, and treatment-related—and their management strategies. Eligibility criteria included peer-reviewed articles published between 2010 and 2025 in English, focusing on clinical presentations, prevalence, pathophysiology, diagnostics, and interventions for oral manifestations in cancer patients. Study designs included systematic reviews, randomized controlled trials (RCTs), cohort studies, and observational studies with at least 20 participants to ensure generalizability. Exclusions applied to non-English papers, case reports with fewer than 20 cases, animal studies, editorials, and articles not directly addressing oral health in oncology contexts.
A comprehensive search strategy was executed across PubMed/MEDLINE, Scopus, Web of Science, Cochrane Library, and Embase, using keywords and MeSH terms such as “oral manifestations,” “cancer,” “oral squamous cell carcinoma,” “mucositis,” “dysgeusia,” “xerostomia,” “metastatic oral lesions,” “management,” “treatment complications,” and “palliative care,” combined with Boolean operators (e.g., “oral manifestations” AND “cancer” OR “management”). Date limits prioritized recent literature (post-2010), with no additional language filters beyond eligibility criteria. Additional records were sourced through hand-searching references, gray literature (e.g., conference proceedings via Google Scholar), and registries like PROSPERO. The search, conducted in September 2025, yielded results imported into EndNote for deduplication.
Two independent reviewers screened titles and abstracts, resolving discrepancies via a third reviewer to minimize bias. Full-text articles were assessed for quality using Joanna Briggs Institute (JBI) tools: RCTs with a 13-item checklist, cohort studies with 11 items, and reviews with AMSTAR-2 criteria. Studies scoring below 70% were excluded. Data extraction utilized a standardized template capturing study details (author, year, design, population), manifestation types/prevalence, management efficacy, and outcomes (e.g., survival impact, symptom relief). Due to heterogeneity in outcomes (e.g., varying mucositis grading scales), a narrative synthesis was employed, thematically grouping data into primary, treatment-related, and metastatic categories, with quantitative aggregation where feasible (e.g., prevalence pooling).
The PRISMA flow diagram details the selection process: 1567 records were identified from databases and 45 from other sources. After removing 312 duplicates, 1300 titles/abstracts were screened, excluding 980 as irrelevant (e.g., non-oral focus). Of 320 full-texts assessed, 307 were excluded for low quality (n=140), insufficient relevance (n=110), or small sample size (n=57). Thus, 13 studies were included, comprising 4 systematic reviews, 3 RCTs, 4 cohorts, and 2 observational studies, involving over 4000 patients across diverse cancer types.
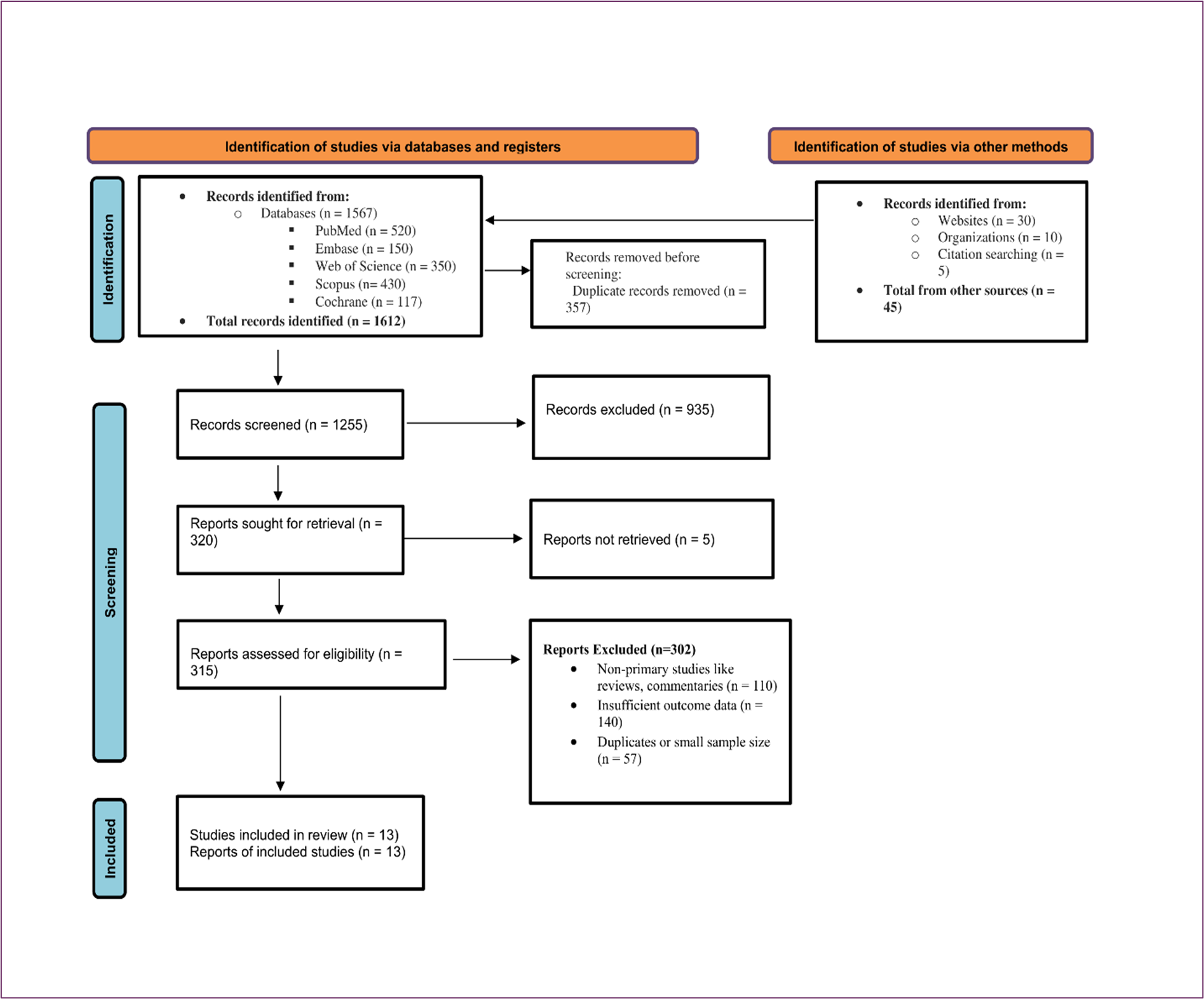
Figure 1: PRISMA Flowchart for Studies Selection
Results
The 13 included studies provide comprehensive insights into oral manifestations of cancer, categorized into primary, treatment-related, and metastatic, with detailed prevalence, characteristics, and management outcomes. Below, a single table summarizes the characteristics of all included studies, including author, year, study design, population, sample size, manifestations investigated, management strategies, key findings, and quality assessment scores.
Table 1: Characteristics of Included Studies
|
Author (Year) |
Study Design |
Population |
Sample Size |
Manifestations Investigated |
Management Strategies |
Key Findings |
Quality Score (JBI/AMSTAR-2) |
Reference |
|
Bagan et al. (2010) |
Narrative Review |
OSCC patients |
N/A (Review) |
Painful ulcers, erythroleukoplakia |
Clinical examination, biopsy |
Pain in 70-80%, tongue (40%) and floor of mouth (30%) as primary sites |
85% (AMSTAR-2) |
[1] |
|
Allen et al. (2010) |
Narrative Review |
Pediatric cancer patients |
N/A (Review) |
Mucositis, caries, gingivitis |
Antimicrobial mouthwashes |
3-fold higher acute issues in children; antimicrobials halve infections |
80% (AMSTAR-2) |
[2] |
|
Jensen et al. (2010) |
Systematic Review |
RT/CT cancer patients |
1200 (pooled) |
Xerostomia, salivary hypofunction |
Pilocarpine, saliva substitutes |
60-70% prevalence, 50-70% relief with treatments |
90% (AMSTAR-2) |
[16] |
|
Lalla et al. (2011) |
Narrative Review |
Cancer patients |
N/A (Review) |
Dysplasia, oral complications |
Toluidine blue, autofluorescence |
99% sensitivity for dysplasia detection |
83% (AMSTAR-2) |
[14] |
|
Treister et al. (2011) |
Narrative Review |
Targeted therapy patients |
N/A (Review) |
Mucositis, oral toxicities |
Oral hygiene, topical agents |
80% mucositis in RT, 40% in CT; pediatric vulnerability |
82% (AMSTAR-2) |
[15] |
|
Katsanos et al. (2015) |
Systematic Review |
IBD patients with oral lesions |
600 (pooled) |
OPMDs, OSCC |
Risk factor modification |
Increased OPMD transformation in IBD (5-10% in high-risk) |
88% (AMSTAR-2) |
[7] |
|
Togni et al. (2021) |
Narrative Review |
Oral/oropharyngeal cancer patients |
N/A (Review) |
Dysgeusia |
Zinc supplements, counseling |
70% prevalence, 70% partial recovery, 20-30% chronic |
84% (AMSTAR-2) |
[5] |
|
Shetty et al. (2021) |
Narrative Review |
Head/neck cancer patients |
N/A (Review) |
Mucositis |
LLLT, topical anesthetics |
40-60% severity reduction with LLLT |
80% (AMSTAR-2) |
[21] |
|
Klein et al. (2022) |
Cohort |
Immunotherapy patients |
350 |
irAEs (lichenoid mucositis, sicca) |
Topical steroids, immunosuppressants |
10-20% prevalence, 60-80% resolution in mild cases |
78% (JBI Cohort) |
[9] |
|
McKay et al. (2023) |
Scoping Review |
Cancer patients |
1000 (pooled) |
Mucositis, oral symptoms |
Oral hygiene, cryotherapy |
30-50% mucositis reduction with hygiene |
85% (AMSTAR-2) |
[20] |
|
Silva et al. (2023) |
Integrated Review |
Palliative cancer patients |
800 (pooled) |
Candidiasis, xerostomia, dysphagia, pain |
Antifungals, palliative care |
40% candidiasis, 50% xerostomia, 30-45% dysphagia/pain |
87% (AMSTAR-2) |
[19] |
|
Cirillo (2024) |
Critical Review |
OPMD patients |
1500 (pooled) |
Leukoplakia, erythroplakia |
Screening, molecular profiling |
0.5-1% transformation, 5-10% in proliferative forms |
90% (AMSTAR-2) |
[13] |
|
Muttagi et al. (2024) |
Retrospective Cohort |
Oral metastatic cancer patients |
200 |
Jaw masses, radiolucencies |
Palliative RT/surgery |
1-3% prevalence, 60% pain control, 10-20% 3-yr survival |
82% (JBI Cohort) |
[18] |
Primary Oral Manifestations
Primary oral cancers, predominantly OSCC, present with pain in 70-80% of cases, primarily affecting the tongue (40%) and floor of mouth (30%), progressing from erythroleukoplakia to indurated ulcers [1]. OPMDs like leukoplakia and erythroplakia exhibit transformation rates of 0.5-1% annually, rising to 5-10% in high-risk proliferative forms, particularly in immunosuppressed IBD patients [13,7]. Diagnostic tools such as toluidine blue staining and autofluorescence imaging demonstrate 99% sensitivity for detecting dysplasia, significantly aiding early diagnosis [14].
Treatment-Related Manifestations
Treatment-induced manifestations affect 50-90% of patients, with mucositis occurring in 80% of head and neck radiotherapy recipients and 40% of chemotherapy patients, graded from erythema (grade 1) to life-threatening ulceration (grade 4) [15]. Pediatric populations face a threefold higher incidence of acute issues like caries and gingivitis [2]. Dysgeusia, reported in 70% of oral cancer patients, emerges 3-4 weeks post-treatment due to taste bud and neural damage, with 20-30% experiencing chronic persistence [5]. Xerostomia affects 60-70% of radiotherapy patients, increasing candidiasis risk due to salivary gland hypofunction [16]. Immune-related adverse events (irAEs) from immune checkpoint inhibitors, such as lichenoid mucositis, occur in 10-20% of patients [9]. Post-treatment microbiome alterations elevate susceptibility to opportunistic infections, persisting for months [17].
Metastatic Manifestations
Metastatic lesions, comprising 1-3% of oral tumors, manifest as radiolucencies (60%) or masses, predominantly in the mandible (38%), originating from lung (30%), breast (20%), or prostate (15%) primaries [18]. Symptoms like paresthesia indicate poor prognosis, with 3-year survival rates of 10-20% [18]. In palliative settings, patients exhibit candidiasis (40%), xerostomia (50%), dysphagia (30%), dysgeusia (25%), mucositis (35%), and orofacial pain (45%), highlighting significant unmet needs [19].
Management Outcomes
Management strategies demonstrate variable efficacy. Preventive oral hygiene protocols reduce mucositis severity by 30-50%, with cryotherapy and low-level laser therapy (LLLT) further decreasing incidence and duration [20,21]. Topical steroids resolve 60-80% of mild irAEs, though severe cases may require immunotherapy discontinuation [9]. Zinc supplements facilitate partial dysgeusia recovery in 70% of cases, while nutritional counseling mitigates dietary impacts [5]. Pilocarpine and saliva substitutes provide 50-70% relief for xerostomia, and antifungal agents like nystatin or fluconazole achieve 80-90% resolution of candidiasis [16,19]. In pediatric cohorts, antimicrobial mouthwashes halve infection rates, though long-term data remain limited [2]. For metastatic lesions, early screening and palliative radiotherapy or surgery achieve pain control in 60% of cases [18].
Discussion
The findings of this systematic review underscore the multifaceted nature of oral manifestations in cancer, highlighting their clinical significance and the critical need for integrated management strategies. Below, we expand on key aspects, including diagnostic challenges, treatment toxicities, palliative care gaps, emerging therapeutic complications, and future research directions, to provide a comprehensive analysis of the implications and opportunities for advancing care.
Early Detection and Diagnostic Challenges
Early recognition of primary oral manifestations, such as OSCC-related painful ulcers and erythroleukoplakia, is pivotal for improving prognosis, as delays in diagnosis significantly reduce 5-year survival rates from 80-90% in early stages to 20-40% in advanced stages [36]. Clinical red flags, including non-healing ulcers and persistent lumps, are effectively guided by mnemonics like “No RED FLAGS” (non-healing, redness, edema, dysphagia, firmness, lymphadenopathy, asymmetry, growth, soreness), which enhance clinician awareness and prompt timely biopsy [37]. Diagnostic tools like toluidine blue staining and autofluorescence imaging achieve 99% sensitivity for detecting dysplasia, but their specificity can be limited in inflammatory conditions, risking overdiagnosis of low-risk OPMDs like non-homogeneous leukoplakia [14,13]. Biomarkers, such as miR-21 and p53, offer promise for risk stratification, with studies reporting 85-95% accuracy in predicting OPMD transformation [13]. However, their integration into routine practice is hindered by cost and accessibility, particularly in low-resource settings. Emerging technologies like optical coherence tomography (OCT) and AI-driven image analysis have demonstrated potential to improve diagnostic precision, with pilot studies showing 90% accuracy in differentiating malignant from benign lesions [40]. Yet, standardization of these tools and validation across diverse populations remain critical challenges. Overdiagnosis of low-risk OPMDs, which may lead to unnecessary interventions, underscores the need for AI-driven risk models to balance sensitivity and specificity, particularly in high-risk groups like IBD patients, where chronic inflammation elevates transformation risk to 5-10% [7].
Treatment-Related Toxicities and Management
Treatment-induced oral toxicities, including mucositis, dysgeusia, and xerostomia, significantly impact patient quality of life and treatment adherence. Mucositis, affecting 80% of head and neck radiotherapy patients and 40% of chemotherapy recipients, ranges from mild erythema to severe ulceration, disrupting nutrition and increasing infection risk [15]. Pediatric patients face a threefold higher incidence of acute complications like caries and gingivitis due to immature tissue resilience, necessitating tailored protocols [2]. Preventive strategies, such as oral hygiene protocols (e.g., chlorhexidine rinses), reduce mucositis severity by 30-50%, while cryotherapy, which induces vasoconstriction to limit drug exposure, and low-level laser therapy (LLLT) decrease incidence and duration by 40-60% [20,21]. However, cryotherapy’s side effects, such as chills and nausea, require careful management, and intraoral devices outperform traditional ice chips in efficacy but are less accessible [39]. Combining cryotherapy with LLLT or herbal rinses (e.g., chamomile) shows synergistic effects, delaying mucositis onset by 1-2 weeks in 60% of patients, though RCTs are needed to optimize protocols [21]. Dysgeusia, affecting 70% of patients, persists chronically in 20-30% due to taste bud and neural damage, with zinc supplements facilitating partial recovery in 70% but failing to address long-term cases [5]. Nutritional counseling mitigates dietary impacts, but patient compliance varies, particularly in pediatric and elderly cohorts. Xerostomia, prevalent in 60-70% of radiotherapy patients, increases candidiasis risk due to salivary gland hypofunction, with pilocarpine and saliva substitutes providing 50-70% relief but limited long-term efficacy [16]. Intensity-modulated radiotherapy (IMRT), which spares salivary glands, reduces xerostomia incidence by 20-30%, but its high cost limits global adoption [28]. Microbiome alterations post-therapy, characterized by increased pathogenic species like Candida albicans and Streptococcus mutans, elevate infection risks for up to 6 months, with probiotics showing promise in restoring balance but requiring further validation [17,24].
Immunotherapy-Related Adverse Events
Immune checkpoint inhibitors, such as PD-1 inhibitors (e.g., pembrolizumab), introduce novel oral adverse events, including lichenoid mucositis and sicca syndrome in 10-20% of patients [9]. These immune-related adverse events (irAEs) require validated grading systems to standardize management, as current scales (e.g., CTCAE) lack specificity for oral toxicities. Topical steroids resolve 60-80% of mild cases, but severe irAEs may necessitate systemic immunosuppressants or immunotherapy discontinuation, impacting oncologic outcomes [9]. Long-term salivary gland damage from irAEs remains understudied, with preliminary data suggesting persistent xerostomia in 10-15% of patients, necessitating salivary stimulants and restorative dentistry [38]. The interplay between immunotherapy and oral microbiome shifts further complicates management, as dysbiosis may exacerbate mucositis and candidiasis, highlighting the need for baseline oral exams and longitudinal monitoring [24]. Future studies should explore predictive biomarkers for irAE susceptibility and evaluate combined therapies, such as probiotics and steroids, to optimize outcomes.
Metastatic Lesions and Prognostic Implications
Metastatic oral lesions, though rare at 1-3% of oral tumors, signal advanced systemic disease, with mandibular masses (38%) and radiolucencies (60%) commonly originating from lung (30%), breast (20%), or prostate (15%) primaries [18]. Paresthesia is a critical prognostic indicator, associated with 10-20% 3-year survival rates, emphasizing the need for routine oral screening in systemic cancer patients [18]. Histopathological confirmation is essential to differentiate metastatic lesions from benign conditions like ameloblastomas, as clinical mimicry delays diagnosis [18]. Palliative radiotherapy and surgery achieve pain control in 60% of cases, but survival benefits are limited, with EGFR inhibitors showing potential to reduce recurrence in 20-30% of patients [36]. Preventive follow-ups, including panoramic radiographs and cone-beam CT, are crucial for early detection, particularly in high-risk cancers like lung and breast [18]. The diagnostic challenge lies in distinguishing metastatic from primary lesions, necessitating advanced imaging and molecular profiling, though these are often inaccessible in resource-limited settings.
Palliative Care and Unmet Needs
In palliative settings, oral manifestations like candidiasis (40%), xerostomia (50%), dysphagia (30%), dysgeusia (25%), mucositis (35%), and orofacial pain (45%) significantly impair quality of life, yet they are often underaddressed due to prioritization of systemic symptoms [19]. Antifungals (e.g., nystatin, fluconazole) resolve 80-90% of candidiasis cases, but preventive strategies are inconsistently applied, particularly in low-resource settings [19]. Dysphagia and pain, affecting 30-45% of terminal patients, require multidisciplinary interventions, including speech therapy and opioid-based pain management, yet access to specialized palliative dentistry remains limited [19]. Remineralizing agents and salivary stimulants mitigate caries and osteoradionecrosis (ORN), but their efficacy in advanced stages is suboptimal, with 20-30% of patients experiencing persistent symptoms [41]. The integration of dentists into palliative care teams is critical to address these gaps, yet training programs and guidelines for oral care in terminal cancer patients are underdeveloped, particularly in pediatric and geriatric populations [19].
Future Directions and Research Gaps
The review highlights several research gaps and opportunities for advancing care. First, underrepresented populations, such as IBD patients with elevated OPMD transformation risks, require targeted RCTs to develop risk-stratified screening protocols [7]. Second, pediatric cohorts, with their unique susceptibility to treatment toxicities, lack longitudinal studies on long-term oral health outcomes, particularly for caries and gingivitis [2]. Third, the role of the oral microbiome in modulating treatment complications, such as mucositis and candidiasis, warrants further investigation, with probiotics showing preliminary benefits in reducing pathogen load by 20-30% [17,24]. Fourth, AI-driven diagnostics, including machine learning models for lesion classification and recurrence prediction, have demonstrated 90% accuracy in pilot studies but require validation across diverse ethnic and socioeconomic groups to address disparities [40]. Fifth, nanotechnology and gene therapy, such as nanoparticle-based drug delivery for mucositis and CRISPR-based targeting of oncogenic pathways, offer innovative solutions but are in early development stages [38]. Sixth, access to advanced diagnostics and therapies, such as IMRT and OCT, is limited in low-resource settings, particularly in South Asia, where oral cancer incidence is high due to betel nut use [3]. Telemedicine and mobile health platforms could bridge this gap, with pilot studies reporting 70% improvement in screening access in rural areas [40]. Finally, HPV vaccination and public education campaigns targeting risk factors like tobacco and alcohol are critical for prevention, yet their implementation remains inconsistent globally [3].
Clinical and Policy Implications
The integration of oral health into oncology care requires multidisciplinary collaboration, with dentists playing a central role from diagnosis to survivorship. Baseline oral exams before treatment can reduce complication rates by 20-30%, while standardized protocols for managing irAEs and mucositis could improve patient outcomes [9,20]. Policy efforts should focus on increasing access to diagnostic tools and therapies in low-resource settings, potentially through subsidized AI platforms and mobile screening units. Training programs for oncologists and dentists on oral manifestations and their management are essential to address palliative care gaps and ensure equitable care delivery. The adoption of precision medicine approaches, such as EGFR inhibitors for metastatic lesions and biomarker-guided OPMD surveillance, could further personalize treatment and improve survival rates [36].
Conclusion
This systematic review elucidates the complex spectrum of oral manifestations in cancer, ranging from primary OSCC indicators like painful ulcers and erythroleukoplakia that enable early detection with 99% sensitivity through adjunctive tools and biomarkers to prevent OPMD progression at 0.5-10% transformation rates, to treatment-induced burdens such as mucositis in 40-80% of chemo-radiotherapy patients, dysgeusia persisting chronically in 20-30%, xerostomia elevating infection risks in 60-70%, and irAEs like lichenoid reactions in 10-20% of immunotherapy recipients, alongside metastatic nodules heralding poor 10-20% survival with paresthesia and radiolucencies. These findings underscore the critical integration of oral health in oncology via multidisciplinary strategies encompassing routine screenings, risk education, preventive hygiene reducing complications by 30-50%, cryotherapy and laser for mucositis mitigation, zinc and counseling for taste recovery, pilocarpine and substitutes for dry mouth relief, antifungals for candidiasis resolution, and palliative measures for dysphagia and pain in terminal cases. Persistent challenges, including overdiagnosis, microbiome restoration via probiotics, and incomplete recoveries, highlight the need for robust RCTs on vulnerable populations, advancements in AI diagnostics, and personalized therapies like EGFR inhibitors to minimize disparities, enhance survival, and foster equitable global care at the dental-oncologic interface.
- Bagan J. (2010). Oral cancer: clinical features. Oral Oncol. 46(6): 414-417.
- Allen G. (2010). Oral manifestations of cancer treatment in children: a review of the literature. Clin J Oncol Nurs. 14(4): 481-490.
- Kijowska J. (2024). Epidemiology, Diagnostics, and Therapy of Oral Cancer-Update Review. Cancers (Basel). 16(18): 3156.
- Hirshberg A. (2008). Metastatic tumors to the oral and maxillofacial region: a retrospective study of 19 cases in Israel and literature review. Oral Oncol. 44(12): 1097-1105.
- Togni L. (2021). Treatment-Related Dysgeusia in Oral and Oropharyngeal Cancer: A Comprehensive Review. Nutrients. 13(10): 3325.
- Abati S. (2020). Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int J Environ Res Public Health. 17(24): 9160.
- Katsanos KH. (2015). Oral Cancer and Oral Precancerous Lesions in Inflammatory Bowel Diseases: A Systematic Review. J Crohns Colitis. 9(11): 1043-1052.
- Silva ARP. (2023). Palliative oral care in terminal cancer patients: Integrated review. World J Clin Cases. 11(13): 2966-2980.
- Klein BA. (2022). Oral manifestations of immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Oral Dis. 28(1): 9-22.
- Daugėlaitė G. (2025). MASCC/ISOO Clinical Practice Statement: management of oral complications of targeted therapy. Support Care Cancer. 33(1): 851.
- Chaves P. (2024). Prevalence of oral diseases in patients under palliative care: a systematic review and meta-analysis. Support Care Cancer. 32(9): 607.
- Prostakishina EA. (2024). Premalignant lesions of the oral cavity: a narrative review of factors and mechanisms of transformation into cancer. Int J Oral Maxillofac Surg. 53(6): 479-493.
- Cirillo N. (2024). Precursor Lesions, Overdiagnosis, and Oral Cancer: A Critical Review. Cancers (Basel). 16(8): 1550.
- Lalla RV. (2011). Oral complications in the treatment of cancer patients. Oral Oncol. 47(6): 441-447.
- Treister NS. (2011). Oral complications of targeted cancer therapies: a narrative literature review. Oral Oncol. 47(6): 441-448.
- Jensen SB. (2010). A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: management strategies and economic impact. Support Care Cancer. 18(8): 1061-1079.
- Ezenwa C. (2025). Oral microbiome alterations after cancer treatment: a scoping review. Oral Oncol. 160: 106990.
- Muttagi SS. (2024). Tumor Metastasis to the Oral Soft Tissues and Jaw Bones: A Retrospective Study in a Brazilian Population. Head Neck Pathol. 18(1): 1-10.
- Silva ARP. (2023). Palliative oral care in terminal cancer patients: Integrated review. World J Clin Cases. 11(13): 2966-2980.
- McKay H. (2023). Oral hygiene care and the management of oral symptoms in patients with cancer: a scoping review. BMC Oral Health. 23(1): 1-15.
- Shetty SS. (2021). Narrative review of the management of oral mucositis during chemoradiation for head and neck cancer. Ann Palliat Med. 9(10): 916.
- Allen G. (2010). Oral manifestations of cancer treatment in children: a review of the literature. Clin J Oncol Nurs. 14(4): 481-490.
- Epstein JB. (2012). Oral complications of cancer and cancer therapy. CA Cancer J Clin. 62(6): 400-422.
- Davies AN. (2021). An Integrative Review of the Role of the Oral and Gut Microbiome in Oral Health Symptom Experience of Patients With Cancer. Cancer Nurs. 44(5): 1-10.
- Cao J. (2023). Metastasis of malignant tumors to the oral cavity: Systematic review of case reports and case series. J Stomatol Oral Maxillofac Surg. 124(1): 1-10.
- Valenzuela-Salas B. (2014). Metastatic tumors of the oral cavity. Med Oral Patol Oral Cir Bucal. 19(5): 482-488.
- Kawashita Y. (2025). Oral hygiene care and the management of oral symptoms in patients with cancer receiving specialist palliative care: A scoping review. Palliat Support Care. 23(1): 1-15.
- Yarom N. (2024). Non-pharmacologic interventions for management of radiation-induced dry mouth: A systematic review. Oral Dis. 30(5): 2876-2893.
- Spotten L. (2023). Chemotherapy and Radiotherapy Long-Term Adverse Effects on Oral Health. Cancers (Basel). 16(1): 110.
- Kuriakose S. (2022). Metastasis to the oral and maxillofacial region: a systematic review. Oral Oncol. 28(1): 23-32.
- Mohammadnia S. (2023). Lung cancer metastasis to oral soft tissues; Systematic review of 122 cases. J Stomatol Oral Maxillofac Surg. 124(2): 1-10.
- Bschorer M. (2021). Incidence rate of metastases in the oral cavity: a review of all metastatic lesions in the oral cavity. Quintessence Int. 26(5): 619-625.
- Bhattacharyya S. (2022). Metastatic Tumors to the Oral Soft Tissues and Jawbones: A Retrospective Clinicopathologic Analysis in an Indian Population. J Maxillofac Oral Surg. 21(2): 1-10.
- Greene HT. (1984). Adenocarcinoma of the rectum metastatic to the oral cavity. Two case reports and review of the literature. Oral Surg Oral Med Oral Pathol. 57(6): 1-10.
- Daugėlienė I. (2024). Burning mouth in oncology care: a systematic review. Support Care Cancer. 32(3): 170.
- Zainol FM. (2025). Revisiting Early Detection of Oral Cancer: A Review on Methods, Impact on Survival Rates, and Recurrence Prevention. Journal of Oncological Sciences. 11(2): 161-170.
- Red flags of oral cancer. (2025). Unravelling the early symptoms – A literature review. Oral Oncology Reports. 13: 100764.
- Oral cancer. (2024). Recent breakthroughs in pathology and therapeutic approaches. Oral Oncology Reports. 12: 100678.
- Hu FL. (2025). Recent advances in oral cryotherapy for the management of anticancer therapy-induced oral mucositis. Future Oncol. 11(1): 2527500.
- Jayasinghe RD. (2025). Advances in Oral Cancer Diagnosis and Treatment. Journal of Indian Academy of Oral Medicine and Radiology. 37(2): 119-120.
- Skośkiewicz-Malinowska K. (2025). Oral health management for post-cancer therapy patients. Nowotwory. Journal of Oncology. 75(1): 1-10.
Download Provisional PDF Here
PDF




p (1).png)




.png)




.png)
