Review Article
Advanced Nanofiber Mesh Technology for Clinical Blood Detoxification: A New Frontier in Translational Nanomedicine
- Gnanendra Sai Kumar Nareboina
Corresponding author: Gnanendra Sai Kumar Nareboina, Doctor of Pharmacy, Department of Pharmacy Practice, GIET School of Pharmacy, Rajamahendravaram, 533296, Andhra Pradesh, India. ORCID ID: 0009-0003-1919-7026
Volume: 2
Issue: 3
Article Information
Article Type : Review Article
Citation : Gnanendra Sai Kumar Nareboina, Poojitha Bandaru, Uma Bekkam, Reena Varshitha Saripalli, Keerthi Vallapureddy, Satya Jagadeesh Botta, Girish Kombathula, Pavan Kumar Pothanapalli. Advanced Nanofiber Mesh Technology for Clinical Blood Detoxification: A New Frontier in Translational Nanomedicine. Journal of Medical and Clinical Case Reports 2(3).
Copyright: © 2025 Gnanendra Sai Kumar Nareboina. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.61615/JMCCR/2025/AUG027140807
Publication History
Received Date
17 Jul ,2025
Accepted Date
30 Jul ,2025
Published Date
07 Aug ,2025
Abstract
Recent developments in nanotechnology have opened new avenues in clinical blood detoxification by overcoming the limitations of traditional methods such as hemodialysis, hemodiafiltration, plasmapheresis, and hemoperfusion. Among these innovations, nanofiber mesh technology stands out for its exceptional surface area, high porosity, and functional versatility, enabling targeted removal of toxins, including protein-bound solutes and uremic toxins, without disrupting essential biomolecules. Electrospun nanofiber meshes, composed of biocompatible polymers and functionalized with materials such as activated carbon, zeolites, and metal-organic frameworks, demonstrate enhanced adsorption efficiency and hemocompatibility. Their ability to integrate selective binding mechanisms, combined with minimal infrastructure requirements, positions them as a promising next-generation solution for extracorporeal blood purification. While most evidence remains preclinical, early in vitro and in vivo models have shown favorable performance in terms of safety, detoxification efficacy, and hemocompatibility. This article highlights the mechanisms, fabrication techniques, clinical potential, and future directions of nanofiber-based blood detox systems, laying a foundation for their translational application in managing critical conditions such as acute poisoning, renal failure, and inflammatory syndromes.
Keywords: Nanofiber Mesh; Blood Detoxification; Electrospinning; Toxin Adsorption.
►Advanced Nanofiber Mesh Technology for Clinical Blood Detoxification: A New Frontier in Translational Nanomedicine
Gnanendra Sai Kumar Nareboina1*, Poojitha Bandaru1, Uma Bekkam2, Reena Varshitha Saripalli1, Keerthi Vallapureddy1, Satya Jagadeesh Botta2, Girish Kombathula2, Pavan Kumar Pothanapalli2
1Research Scholar, Department of Pharmacy Practice, GIET School of Pharmacy, Rajahmundry, Andhra Pradesh, 533296, India.
2Research Scholar, Department of Pharmaceutical Sciences, GIET School of Pharmacy, Rajahmundry, Andhra Pradesh, 533296, India.
Introduction
Blood detoxification is a critical clinical intervention aimed at eliminating harmful substances such as toxins, drugs, and metabolic byproducts from the bloodstream, particularly in cases of poisoning, metabolic imbalances, or specific systemic diseases. Traditional methods like hemodialysis, hemodiafiltration, plasmapheresis, and hemoperfusion have long been used in clinical practice to facilitate extracorporeal toxin removal. However, these conventional techniques often present limitations such as incomplete clearance of certain compounds and risks, including coagulation, infection, and biocompatibility issues [1]. Recent advances in surface engineering and nanotechnology, particularly nanofiber mesh materials that offer promising alternatives to these limitations. Owing to their high surface area, structural tunability, and selective binding capabilities, nanofiber meshes can be engineered to remove a wide spectrum of toxins more effectively and safely. Unlike traditional filters, they provide enhanced interaction with target molecules and may significantly improve therapeutic outcomes. The development and clinical translation of nanofiber mesh technology could revolutionize detoxification protocols, offering novel solutions for acute and chronic conditions requiring blood purification [2].
Conventional Clinical Blood Detoxification Methods
Conventional blood purification methods remain the clinical standard for managing various toxic and metabolic conditions. These techniques rely on physical principles such as diffusion, convection, and adsorption etc to remove toxins from blood, and are widely used despite their limitations. The following subsections provide a concise overview of key conventional methods, including their procedural flow and inherent drawbacks.
Hemodialysis (HD)
Hemodialysis is one of the oldest and most proven ways of blood purification, which is regularly applied for the treatment of patients with kidney disease. This is done by pumping the patient’s blood through a machine that separates out poisons and extra water. The patient under dialysis has body fluids as electrolytes, water, and other smaller molecules, such as urea and creatinine, removed through the use of a semi-permeable membrane in the dialysis machine. Although very effective with small-molecule toxins or other small molecular weight substances, it is not very effective with large toxins that are protein-bound. The mechanism of solute removal via diffusion across membranes is shown in Fig. 1 [3].
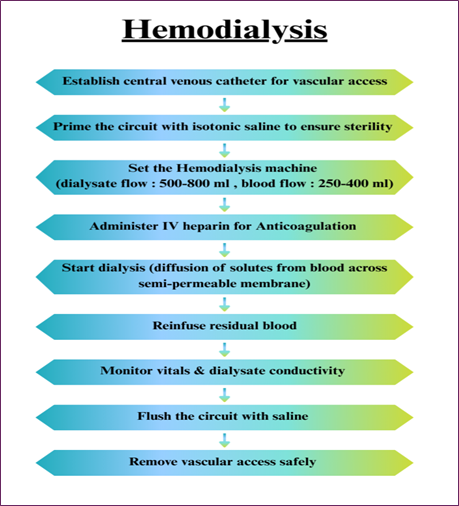
Fig. 1. Hemodialysis process flow showing solute diffusion across a semi-permeable membrane.
Limitations
- The large biomolecules, such as proteins and lipids, can’t be effectively removed.
- This may cause hypotension, nausea, blood clot formation, and muscle cramps during the process.
- This requires frequent sessions at least three times per week [4,12].
Hemodiafiltration (HDF)
An example of the other blood purification process is Hemodiafiltration, which is a technique for filtering blood that is frequently applied in critical care practice and essential for patients with AKI (Acute Kidney Injury). In this method, a membrane separates blood and the filtrate is replaced by a sterile solution. Hemodiafiltration has been preferred over hemodialysis because it can filter out larger molecular-sized substances. Nevertheless, it has some shortcomings in properly addressing some toxins with a great potential for fast interaction. Combined convective and diffusive toxin clearance in hemodiafiltration is outlined in Fig. 2 [5].
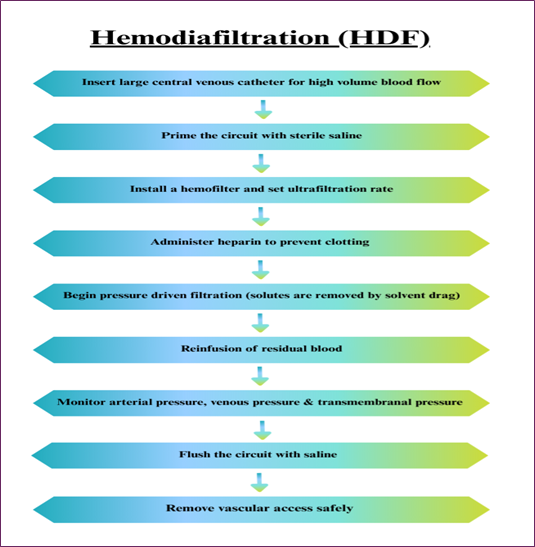
Fig. 2. Hemodiafiltration flowchart illustrating combined convective and diffusive toxin removal.
Limitations
- This process can only remove water-soluble toxins from the body while other toxins are still within the bloodstream.
- This had a potential risk of developing hypocalcaemia, hypomagnesaemia, hypoglycemia, hypotension & hyponatraemia.
- It is comparatively more costlier than traditional methods [6,12].
Plasmapheresis / Therapeutic plasma exchange (TPE)
In plasmapheresis or therapeutic plasma exchange, the patient’s plasma containing toxins, antibodies, and other dangerous components is withdrawn and replaced with fresh plasma. This technique is most beneficial for autoimmune diseases such as myasthenia gravis or Guillain-Barré syndrome, as well as specific poisonings. However, plasmapheresis is associated with some risks, like allergy, infection, or hemorrhage, and is normally applied only in some cases. It is also used to treat patients suffering from conditions like poisoning, kidney failure, sepsis, and other metabolic disorders. Several methods have been developed and refined over time to enhance the efficacy of blood detoxification. The steps of plasma separation and replacement involved in plasmapheresis are presented in Fig. 3 [7,8].
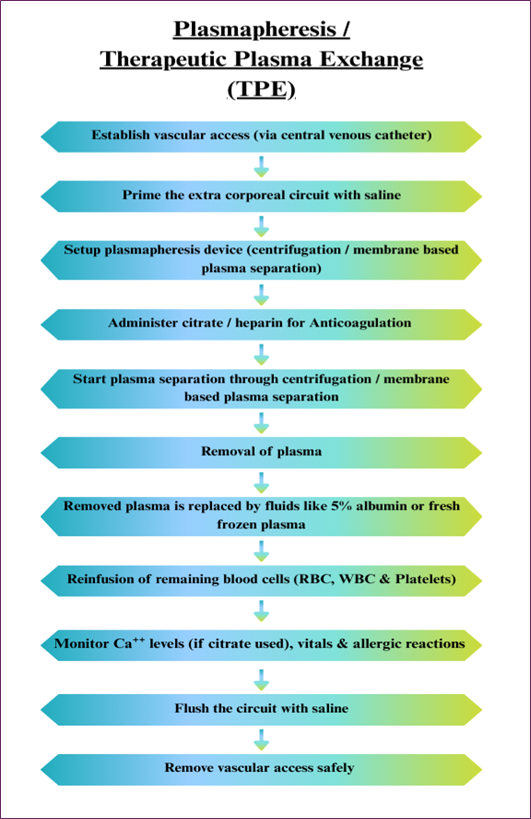
Fig. 3. Plasmapheresis flowchart showing plasma separation and fluid replacement by centrifugation or membrane filtration.
Limitations
- Mainly, it is used in the case of a toxin that binds with the plasma protein, but it has a poor capacity to eliminate free toxins.
- Bleeding complications may arise due to the development of thrombocytopenia.
- There is a possibility of clotting in the adsorbent cartridges for which anticoagulants may be necessary.
- This is not available in many health care facilities because the technology demands special installations [10,12].
Hemoperfusion (HF)
Hemoperfusion is a technique in which the blood is passed over sorbent materials like activated charcoal and resin that adsorbs the toxins from the blood. This technique is especially applied in the management of drug overdose and poisoning, because quick elimination of the toxins is mandatory. Notably, hemoperfusion can effectively remove all small and large molecular toxins that are often difficult to eliminate through the dialysis. Still, it is not popular because of several side effects, such as thrombocytopenia and bleeding. Toxin adsorption during hemoperfusion using sorbent cartridges is illustrated in Fig. 4 [9].
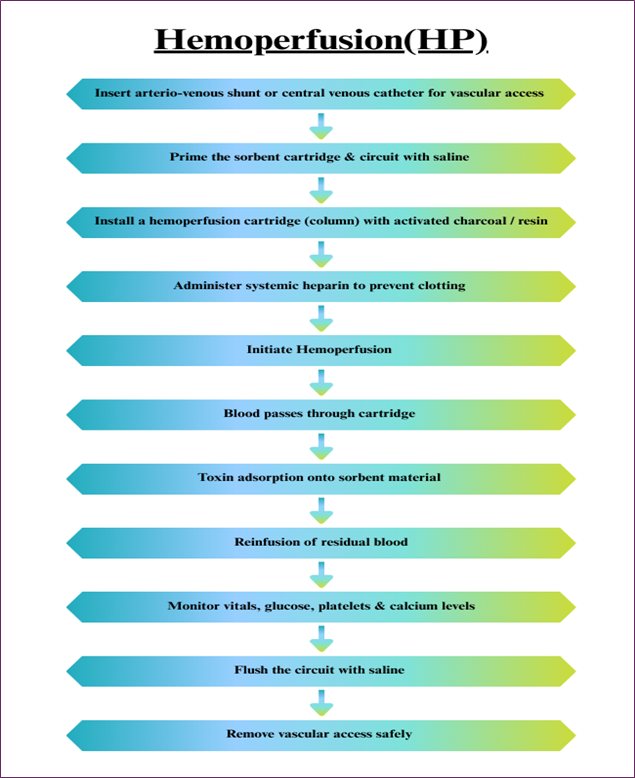
Fig. 4. Hemoperfusion process illustrating toxin adsorption through an activated charcoal or resin cartridge.
Limitations
- Patients may develop allergic reactions, or anaphylaxis, to the replacement fluids used during the process.
- Risk of hypocalcaemia as a result of practices that involve the use of anticoagulant drugs.
- Effective only for single sessions or follow-up appointments for different complaints; could take a longer time and prove expensive for patients with chronic diseases.
- Can result in infection of the catheter site, particularly when the catheter is used for a very long period [11,12].
Challenges and Solutions in Clinical Detoxification
Hemodialysis, hemodiafiltration, plasmapheresis, and hemoperfusion are well-established techniques for removing toxins from the bloodstream. However, each of these conventional approaches presents specific limitations that reduce their overall effectiveness. For instance, hemodialysis struggles to eliminate protein-bound toxins and relies entirely on dialysis solutions for toxin removal. In contrast, hemodiafiltration shows limited capacity in clearing large molecular toxins and typically requires high-pressure systems, which can induce fluctuations in blood pressure [11-13].
Plasmapheresis often necessitates plasma replacement to prevent nutritional loss, making the procedure complex and costly. Moreover, hemoperfusion can trigger inflammation or blood cell damage due to non-biocompatible adsorbents and indiscriminately removes both harmful and beneficial molecules [14,15]. In response to these limitations, nanofiber mesh-based detoxification offers a promising alternative. This technology selectively removes protein-bound toxins without the need for dialysis solutions, thus addressing key shortcomings of hemodialysis. It is capable of eliminating both small and large toxins efficiently, without depending on high-pressure systems, thereby reducing the risk of blood pressure instability common in hemodiafiltration.
Unlike plasmapheresis, nanofiber meshes do not require plasma separation or replacement, simplifying the detoxification process and minimizing associated costs. Additionally, they are engineered with biocompatible materials, which help to prevent blood cell damage and inflammatory responses seen in hemoperfusion. Their selective adsorption capability allows for the removal of only harmful substances while preserving beneficial biomolecules, presenting a safer and more targeted approach to clinical blood detoxification. The challenges of conventional clinical detoxification methods and potential solutions with nanofiber mesh technology were shown in Fig. 5 [16-19].
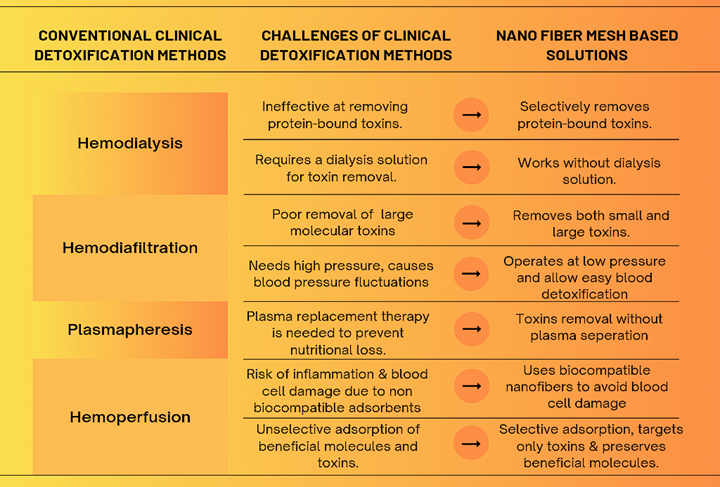
Fig. 5. Challenges of conventional clinical detoxification methods and potential solutions with nanofiber mesh technology.
Advanced Nanofiber Mesh Technology: A Novel Approach
The use of nanofiber mesh in the medical field represents a significant innovation, particularly in blood purification applications. This technology involves the fabrication of ultrafine fibers typically measuring at least one nanometer in diameter using electrospinning techniques that enable precise control over fiber diameter and distribution. Owing to their unique structure, nanofiber meshes offer a high surface area to volume ratio, making them highly effective in filtering undesirable substances (toxins, drugs and metabolic waste products) from the blood stream. This capability positions nanofiber meshes as a promising enhancement to conventional blood detoxification methods like hemodialysis, hemodiafiltration, plasmapheresis, and hemoperfusion [20,21].
One of the most notable features of nanofiber meshes is their adaptability. They can be functionalized to selectively bind specific toxins, thereby improving their detoxification efficiency beyond that of standard filtration approaches. Moreover, their high porosity promotes smooth blood flow, reducing the risk of clotting and infection often associated with traditional methods. Nanofiber materials are also largely biocompatible, minimizing the likelihood of immune responses or adverse foreign body reactions. Overall, nanofiber mesh technology holds the potential to revolutionize clinical blood detoxification, offering a safer and more efficient alternative to existing practices [22,23].
Composition of Nanofiber Mesh for Clinical Blood Detoxification
Nanofiber meshes for blood detoxification are typically fabricated from biocompatible synthetic polymers, often blended with functional adsorbents or coatings to target toxins. Common base polymers include poly (ethylene‑co‑vinyl alcohol) (EVOH), polyethersulfone (PES), polyacrylonitrile (PAN), polycaprolactone (PCL), cellulose acetate (CA), regenerated cellulose, and chitosan or other biopolymers [24-26]. These fibers are often electrospun as nonwoven meshes to maximize surface area. To improve the adsorption of uremic toxins or inflammatory mediators, the nanofibers are frequently combined with inorganic or organic adsorbents. Several nanofiber composites have been engineered to improve toxin removal efficiency. For instance, EVOH-based fibers loaded with activated carbon (AST-120) have been developed to specifically adsorb indoxyl sulfate. Likewise, combining zeolites with EVOH and PES polymers enhances their ability to trap uremic waste. In other studies, PAN fibers coated with metal-organic frameworks like Cu-BTC showed improved urea binding, while functionalized PAN-clay blends were tailored to boost bilirubin clearance by introducing positively charged groups such as lysine. [27,28].
Besides inorganic additives, nanofibers are frequently functionalized chemically or with biological ligands. For instance, PAN fibers grafted with branched polyethyleneimine or lysine introduce cationic binding sites for bilirubin. Regenerated cellulose fibers have been surface-modified with affinity dyes (e.g., Cibacron Blue) to selectively bind bilirubin and other proteins. In advanced designs, multilayer meshes combine different materials: one reported hemodialysis membrane had a dual-layer structure, an inner layer of polydopamine-coated PAN fibers modified with a Cu-BTC MOF, and an outer layer of chitosan/sericin fibers, achieving high urea and creatinine clearance with excellent hemocompatibility. Natural proteins like albumin may also be immobilized on fiber surfaces (e.g., via covalent linkage) to serve as “bait” for protein-bound toxins (as in a 3D nanofiber sponge, where surface-bound BSA enhanced bilirubin uptake). A variety of mesh formulations and their specific toxin targets are compared in (Table 1) [24-30]. In summary, clinically oriented detox meshes typically use biostable polymers (EVOH, PAN, PES, PCL, cellulose, etc.) combined with nanoporous adsorbents (activated carbon, zeolites, MOFs) or functional coatings (e.g. polydopamine, albumin, ionic polymers) to achieve high toxin capture while maintaining structural integrity. Material composition and structural features of nanofiber meshes are detailed in Fig. 6 [24-30].
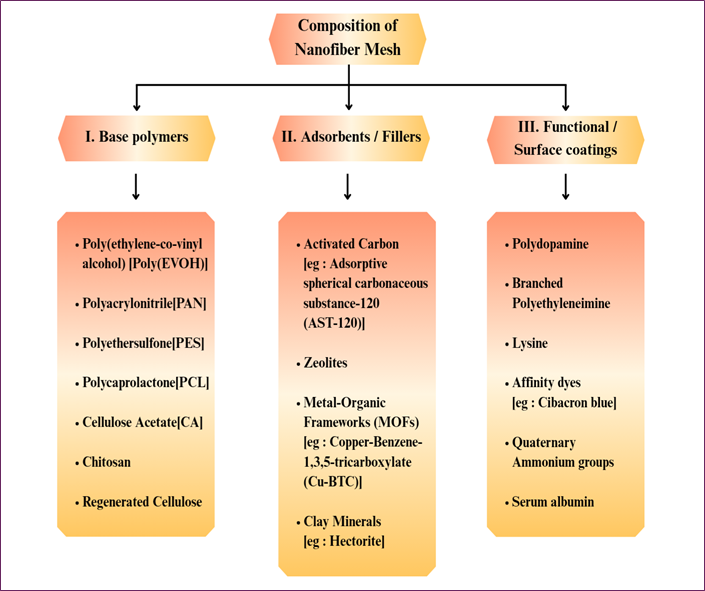
Fig. 6. Structural composition of nanofiber mesh used in blood detoxification, comprising base polymers, adsorbents/fillers, and functional or surface coatings for improved toxin clearance and hemocompatibility.
Nanofiber electrospinning blends are continuously innovated for clinical use. For example, recent research has tailored fiber chemistry via density-functional theory to graft quaternary ammonium groups onto PAN nanofibers, achieving bilirubin adsorption far exceeding that of conventional activated carbon. Biodegradable polymer blends are also explored; one study found that plain electrospun PCL meshes readily adsorb uremic toxin-laden plasma proteins without causing coagulation, though unmodified PCL can trigger immune responses (highlighting the need for biocompatible modifications). In all cases, the mesh composition must balance high adsorptive surface area with blood compatibility. Common thread candidates (e.g., hydrophilic polymers or heparinized layers) are often included to enhance wettability and reduce platelet activation. Thus, nanofiber detox membranes typically feature multicomponent designs, a structural polymer backbone with functional fillers/coatings that capture specific toxins while resisting biofouling [29-32].
|
Polymer Base |
Additive |
Target Toxin |
Mechanism |
|
EVOH |
AST-120 |
Indoxyl sulfate
|
Adsorption |
|
PAN |
Cu-BTC MOF |
Urea |
Metal-ligand complexation
|
|
PAN |
Lysine graft |
Bilirubin |
Electrostatic interaction
|
|
Cellulose
|
Cibacron Blue dye |
Proteins |
Affinity Binding |
|
PCL |
Unmodified |
Plasma proteins |
Hydrophobic interaction
|
Table 1: Comparison of Nanofiber Mesh compositions and their target toxins.
Hemocompatibility of Nanofiber Mesh Technology
For any blood-contacting detox device, compatibility with blood and the immune system is crucial. Recent studies report that optimized nanofiber meshes can be highly hemocompatible. In vitro assays (hemolysis tests, complement activation, platelet adhesion) have shown minimal hemolysis (often <5% even at high fiber concentrations) for functionalized PAN-based fibers [33]. For example, PEI (polyethyleneimine)-grafted PAN membranes exhibited near-zero hemolytic activity and resisted nonspecific protein and blood-cell adhesion, even demonstrating mild anticoagulant effects. Likewise, a calculation-driven PAN nanofiber design (with quaternary ammonium groups) had <5% hemolysis at 10 mg/mL, meeting blood-material safety standards. In a 3D albumin-coated nanofiber sponge, perfusion tests showed large bilirubin uptake with good blood compatibility (no clotting or significant inflammatory response). Other reports note that dual-layer PAN/chitosan-Sericin meshes also exhibited excellent blood compatibility during simulated hemodialysis. Notably, nanofiber membranes often compare favorably or exceed conventional adsorbents: albumin-coated resin (Amberlite XAD-7) showed much lower platelet loss and better cell counts than uncoated resin during perfusion, indicating the value of protein coatings [25,27,32].
Surface chemistry greatly influences these outcomes. Hydrophilic or bioactive coatings (e.g., albumin, heparin, PEG) are widely used to improve compatibility. Albumin-collodion films on adsorbents (inspired by artificial cells) significantly reduce coagulation and leukocyte adhesion, and hemoperfusion materials with albumin coatings (e.g., HSA on XAD-7 beads) have been demonstrated to be blood-compatible and effective in patients [33]. Heparinized or zwitterionic polymer layers on nanofibers also mitigate thrombogenicity and protein fouling. By contrast, unmodified hydrophobic fibers can activate immune pathways: for instance, uncoated PCL meshes triggered a strong humoral response despite not inducing clotting. Hence, most nanofiber detox meshes incorporate charged or PEGylated segments to discourage protein aggregation and complement activation [34-38]. Evaluations of blood compatibility for functionalized nanofiber meshes are shown in (Table 2)
|
Nanofiber Composition |
Functional Modifications |
Hemocompatibility Findings
|
References |
|
PAN |
Quaternary ammonium groups |
<5% hemolysis at 10mg/ml; meets safety standards |
Fu et al.[26] (2024) |
|
PAN |
Albumin-coated (3D sponge) |
Good blood compatibility, no clotting, efficient bilirubin uptake |
Yuan et al.[32] (2018) |
|
PAN |
Polyethyleneimine grafted |
Near-zero hemolysis, blood cell adhesion, and mild anticoagulant effect |
Li et al.[33] (2024) |
|
PAN / Chitosan |
Sericin coating (dual-layer mesh) |
Excellent blood compatibility during hemodialysis |
Li et al.[33] (2024) |
|
XAD-7 Resin |
Albumin coated |
Better platelet retention |
Mahdipour et al.[35] (2022) |
|
PCL |
Uncoated |
Induced a strong humoral immune response |
Zhang et al.[34] (2024) |
Table 2: Hemocompatibility Profiles of Functionalized Nanofiber Meshes for Clinical Detoxification
In Vitro vs In Vivo
Most evidence so far is from bench tests or small animal studies. In vitro blood loop or plasma perfusion experiments generally show that well-designed nanofiber meshes cause no significant hemolysis or thrombosis. One recent calculation-guided PAN adsorbent study explicitly demonstrated that its blood compatibility is sufficient for safe hemoperfusion. In vivo data are still emerging, but existing reports (e.g. animal models of nanofiber implants) suggest minimal inflammatory reaction and good integration when fibers are properly functionalized [26].
Biofouling from proteins or cells remains a concern; accordingly, nanofibers are often tested for protein adsorption. For example, electrospun meshes with high-density cationic sites showed anti-fouling behavior (low nonspecific protein adhesion). Overall, the trend is that modern nanofiber adsorbents, especially those employing anti-thrombogenic coatings, demonstrate excellent hemocompatibility in vitro. Further preclinical and clinical studies will clarify in vivo performance, but early work indicates these meshes can match or exceed the blood-compatibility of legacy dialysis/adsorbent materials [33].
Engineered Blood Detoxification Mechanisms of Nanofiber Mesh Technology
Recent studies show that electrospun nanofiber meshes can remove blood toxins via multiple mechanisms. Nanofibers have extremely high surface area and porosity, providing abundant binding sites. Key detoxification pathways include the following:
Adsorption / Filtration
Toxins physically adsorb onto the fiber surface or get trapped in the porous network. For example, zeolite–polymer composite nanofibers (poly(ethylene-co-vinyl alcohol) matrix with incorporated zeolite) were shown to selectively adsorb small uremic toxins like creatinine. Similarly, activated carbon loaded nanofibers (e.g. EVOH/AST-120 fibers) rapidly adsorb protein-bound uremic solutes (indoxyl sulfate), with diffusion of toxin into the fiber pores being rate-limiting [39-42].
Electrostatic / Chemical Binding
Nanofibers can be functionalized with charged or reactive groups to bind toxins. Studies have demonstrated that combining zeolites with EVOH polymer matrices in nanofiber form enhances the selective capture of small toxins such as creatinine. In a similar approach, nanofibers infused with activated carbon particles have shown rapid binding of protein-associated toxins like indoxyl sulfate, where the movement of toxins into the fiber's porous structure plays a key role in the overall efficiency [26,43].
Affinity / Selective Targeting
Specific ligands can be attached to nanofibers for targeted binding. A prominent example is polymyxin B immobilized on a nanofiber sponge: this ligand binds bacterial endotoxin (LPS) with very high specificity. In one report, PMB-functionalized fibers removed ~99.6% of endotoxin from protein-containing solution versus only ~17% by unmodified fibers. This demonstrates selective toxin capture without excessively depleting serum proteins. Enzymes or antibodies could be similarly grafted to degrade or target specific uremic toxins [44].
Size Exclusion (Microfiltration)
The fiber mesh can physically filter out cells, bacteria, or large complexes. With fiber diameters in the nano- to micron-range, nanofibrous mats act as microfiltration membranes. Although blood filters must preserve cells, nanofibers could remove cellular debris or agglomerates that carry toxins. In other industries, electrospun membranes have been shown to microfilter bacteria or viruses, suggesting similar potential in blood purification [39,44,45]. In summary, nanofiber detox devices combine high porosity and tunable surface chemistry to adsorb a broad range of toxins. The fibers’ large specific surface area and functional groups allow efficient capture via physical adsorption, electrostatic attraction, chelation, and ligand affinity. Multiple pathways enabling selective toxin removal by nanofiber meshes are visualized in Fig. 7 [26,39-45].
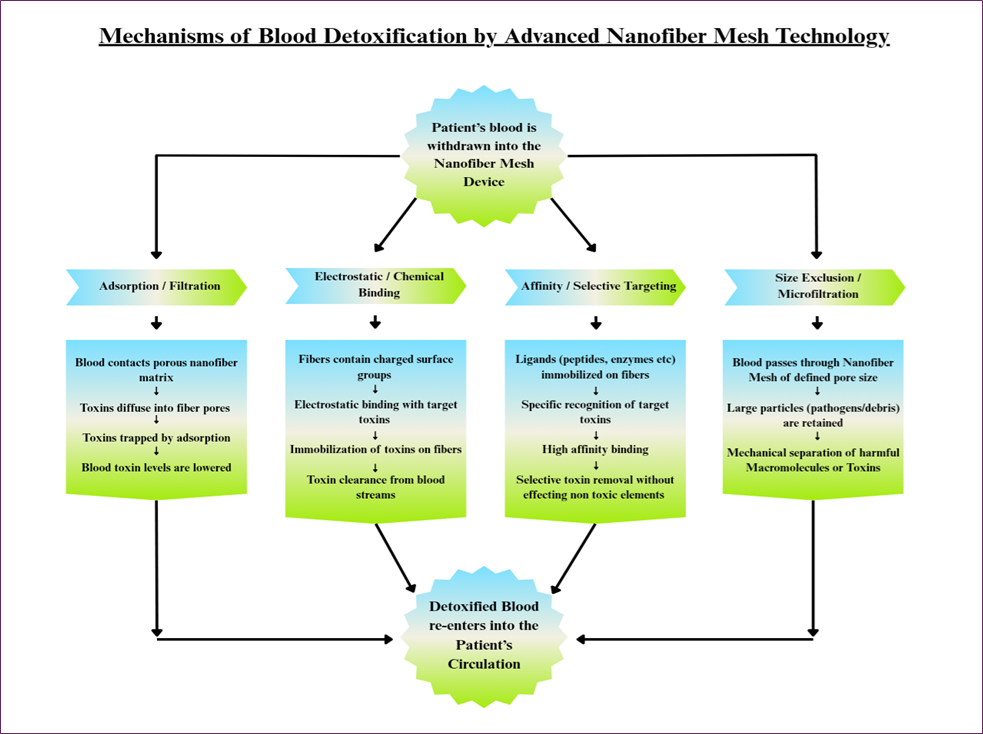
Fig. 7. Mechanisms of blood detoxification by nanofiber mesh technology, showing parallel pathways: adsorption, electrostatic/chemical binding, affinity/selective targeting, and size exclusion, each facilitating selective toxin removal and ensuring safe return of detoxified blood to the patient’s circulation.
Operational Workflow of Nanofiber Mesh-Based Blood Detoxification
- Blood Withdrawal and Arterial Pressure Monitoring
First, blood is removed from the body of the patient, purified, and then returned to the patient by the detoxification process. Blood first goes to the Arterial Pressure Monitor, where it continuously measures blood pressure. It also helps to make sure flow rates are stable and to find abnormalities early.
- Blood Pumping and Heparin Infusion
Then, the blood flows through the Blood Pump and is then propelled forward in the circuit. Heparin Infusion is administered to prevent clotting during the purification process and to keep the blood in a fluid state. It is important because any coagulation in the circuit would block flow and damage the filtration.
- Filtration Unit with Nanofiber Mesh
The Filtration Unit is the heart and soul of the real detoxification process. The series of nanofiber mesh layers contained in this unit serves as ultra-fine filters. Blood flows through these meshes, and toxins and impurities are trapped within the nanofibers. The ability to act as an efficient filter is based on the structure of the nanofibers in which molecules are captured via size, charge, or chemical composition, and ensure blood is purified while allowing essential components to pass through [46-48].
- Air Trap and Air Detection
After passing through the filtration unit, it is opened to the air trap and air detector. This is important to detect and eliminate any blood air bubbles that have entered the blood during filtration. Air embolisms, if not detected, could reach the bloodstream and produce serious complications in the patient. The detector makes absolutely sure that only air-free blood enters the patient.
- Venous Pressure Monitoring
Detoxified blood then reaches the Venous Pressure Monitor site, where it measures pressure before returning to the patient. The pressure at this stage must be monitored to make sure the blood is returning at a safe and level pace.
- Automatic Clamp Safety Mechanism
The blood then passes through an Automatic Clamp. The safety feature is continuously monitoring for abnormal readings, such as pressure changes and air. If there's something improper going on, the clamp immediately stops blood flow to the patient. With this automatic shut-off, there is one more safeguard for the patient in the case of any system malfunction [49,50].
- Restoration of Purified Blood
After all checks are complete, the blood is returned to the patient detoxified of harmful toxins and impurities. This completes the detoxification cycle, keeping the patient's bloodstream clean, minimizing the risk of a toxin-based complication. The clinical flow of blood detoxification using Nanofiber mesh technology is represented in Fig. 8 [46-51].
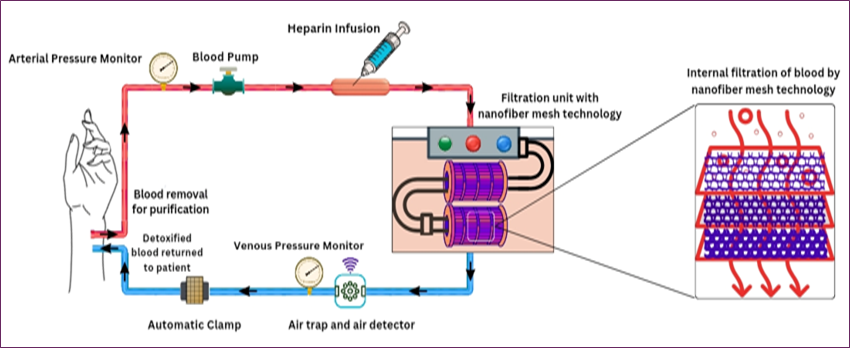
Fig. 8. Schematic of the operational workflow for nanofiber mesh-based blood detoxification, showing key clinical components including blood withdrawal, anticoagulation, filtration through the nanofiber mesh, and return of purified blood.
Electrospinning Fabrication of Nanofiber Mesh for Clinical Detoxification
Electrospinning is a voltage-driven process that relies on electrohydrodynamic instability to fabricate nanofiber meshes and, therefore, is one of the most effective approaches to its production. This makes the technique ideal in clinical detoxification applications because the fibers can be created to be as small as nanometers in diameter or up to micrometers [52]. Compared to other, more complicated methods for creating nanostructures, the process is relatively affordable and can be applied to a wide variety of materials, from polymer solutions to melts. The primary advantage of electrospinning is the possibility to achieve a high degree of uniformity in the fiber diameter, and this is also the main parameter that should be controlled in order to create the nanofiber meshes with the desired functionality for medical applications. The electrospinning process for Nanofiber mesh fabrication is shown in Fig. 9 [53,54]. A standard electrospinning setup consists of three essential components: a power supply, polymer solution, and syringe pump. The syringe pump regulates the amount and rate of polymer solution that is delivered to the sample, and the high-voltage power supply charges the electric field through which the solution is forced through the syringe needle. The electric field acts on the polymer solution to produce thin charged liquid jets that freeze to nano-scale fibers upon contact with a collector. This lays down great accuracy, as well as feasibility for the replication. The electrospinning setup is schematically shown in Fig. 10 [55].
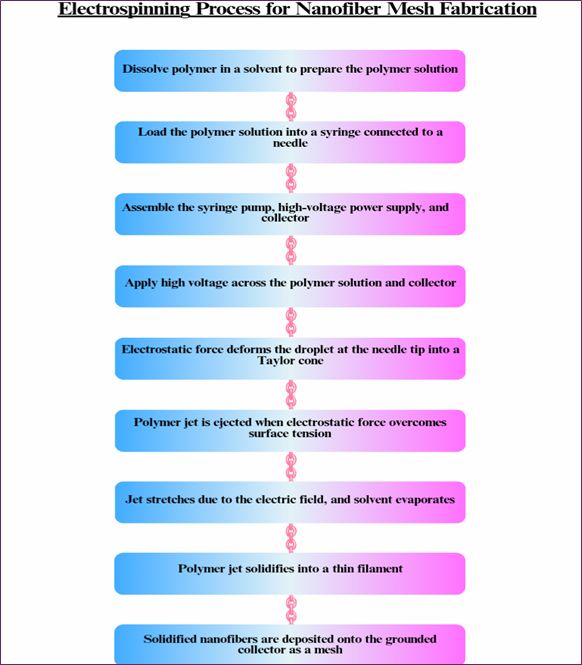
Fig. 9. Flow chart illustrating the step-by-step electrospinning process for fabricating nanofiber mesh designed for clinical blood detoxification applications.
Despite the fact that electrospinning has been widely used than other techniques, which is adopted for the fabrication of nanofiber mesh, whereas the other techniques have been used in clinical detoxification, although to a limited extent. Techniques, including freeze-drying, phase separation, have been employed in the production of nanofiber meshes with distinct properties. Also, other types of electrospinning, like coaxial electrospinning and needle-less electrospinning, have gained interest in the fabrication of different types of fibers. These techniques generally work in synergy with electrospun nanofibers since they resolve the issues that can be faced during the fabrication of the nanofiber meshes for clinical detoxification [56-58].
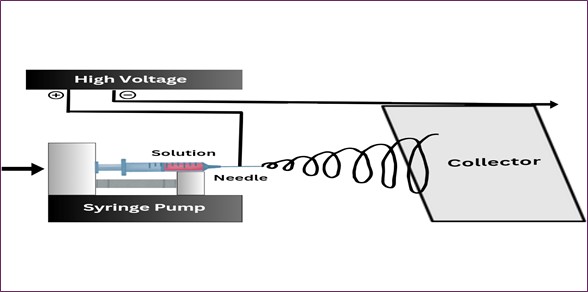
Fig. 10. Schematic representation of the electrospinning setup used for nanofiber mesh fabrication, highlighting key components such as the high-voltage power supply, syringe pump, needle, and collector.
Performance Assessment of Conventional and Nanofiber Mesh-Based Detoxification Techniques Clearance Efficacy for Uremic Toxins
Conventional hemodialysis (HD) efficiently removes small water-soluble toxins (e.g. urea, creatinine). High-flux HD, hemodiafiltration (HDF) further improves the removal of middle molecules (e.g. β₂-microglobulin). For example, direct hemoperfusion (HP) columns (e.g. Lixelle for β₂-m) can reduce serum β₂-microglobulin by 60–79% in a single session. However, conventional dialysis/HDF/HF cannot effectively remove protein-bound uremic toxins (PBUTs). Plasmapheresis (therapeutic plasma exchange, TPE) removes all plasma solutes (including albumin-bound toxins) by wholesale plasma replacement, so it can eliminate large and protein-bound toxins (e.g., auto antibodies, lipoproteins) [59-62].
Nanofiber mesh devices are investigational; one design (poly(EVOH) fiber with embedded zeolite) adsorbs creatinine and urea via porous sorbent. In vitro, the zeolite-polymer fibers achieved ~67% of the free-zeolite adsorption capacity for creatinine. Another design (poly(sodium acrylate) PSA fibers) absorbs excess fluid and may retain urea, but its polymer alone does not adsorb toxins. Overall, nanofiber adsorbents hold promise for the selective removal of specific uremic solutes (e.g. creatinine) without dialysate, but clinical efficacy data are lacking [51,63-66].
Clearance Efficacy for Inflammatory Mediators and Cytokines
Standard HD and HDF filters (with typical pore sizes) remove negligible cytokine mass due to short cytokine half-lives. Hemodiafiltration was theorized to clear cytokines, and modern adsorbent cartridges (e.g. CytoSorb®) are designed to capture mid-sized inflammatory mediators. Emerging data show CytoSorb use lowers IL-6, IL-10, and TNF-α levels in septic shock and may improve hemodynamics. However, rigorous trials have generally not demonstrated superior cytokine clearance vs. controls. Plasmapheresis removes both pro- and anti-inflammatory cytokines as well as complement factors, but one study found no additional cytokine reduction with TPE over standard care in septic shock [66,67].
Hemoperfusion (HP) with endotoxin or cytokine adsorbers can transiently reduce circulating PAMPs/DAMPs; e.g., polymyxin-B columns target endotoxin and have shown improved hemodynamics in some trials, though the survival benefit is unproven. Nanofiber meshes have not been tested for cytokine removal. The biocompatible EVOH-zeolite fibers theoretically do not generate cytokines and could be functionalized with ligands for specific mediators, but this remains experimental. In summary, conventional dialysis methods inadequately clear cytokines without special filters, specialized hemoperfusion adsorbers can reduce cytokine levels, and nanofiber systems offer a potential but unvalidated approach [68,69].
Clearance Efficacy for Drugs and Toxins
Hemodialysis effectively clears small, water-soluble drugs with low protein binding (e.g. lithium, salicylates). Hemodiafiltration similarly removes water-soluble toxins via convection. Plasmapheresis excels at clearing substances with very high protein binding and low volume of distribution, since it eliminates whole plasma components. For example, TPE can remove >80% of a drug dose if it is >90% protein-bound and mostly intravascular. Hemoperfusion (e.g. activated charcoal or resin columns) is a classic therapy for poisoning: it can remove many drugs and toxins irrespective of water solubility, especially if they adsorb onto the sorbent (e.g. theophylline, carbamazepine). Compared to dialysis, hemoperfusion often achieves faster and broader drug clearance. Nanofiber meshes could be tailored with sorbent materials for specific toxins. To date, demonstrated examples focus on uremic solutes; no clinical reports describe drug adsorption by nanofiber filters [66,70].
Biocompatibility
Synthetic dialysis membranes (polysulfone, polyacrylonitrile, PMMA, etc.) are designed for blood compatibility and cause minimal complement activation. In contrast, older cellulosic membranes triggered pronounced complement activation and leukopenia. Modern HF and CRRT filters use similar synthetic materials, so their blood-contact effects are comparable to HD. Plasmapheresis via membrane separation uses very large-pore filters: it removes all plasma but leaves blood cells intact, generally avoiding complement triggering, though it may still stress coagulation by removing clotting factors. Hemoperfusion historically caused adverse blood reactions: early sorbents (charcoal, uncoated resins) induced thrombocytopenia, leukopenia, and complement activation. Today’s adsorbents are engineered for biocompatibility (e.g., polymeric beads, albumin, or heparin-coated surfaces) and often use plasma separation to shield blood cells. Studies show albumin- or cellulose-coated charcoal columns lose markedly fewer platelets than uncoated ones. Nanofiber mesh filters use inherently biocompatible components: for example, poly(ethylene-co-vinyl alcohol) is blood-compatible, and zeolite adsorbents are chemically stable and non-toxic in vitro. However, some nanofiber materials (e.g., poly(sodium acrylate) PSA used for fluid absorption) are not proven safe for blood contact. Overall, nanofiber constructs could offer high surface area with low resistance, but their in vivo blood biocompatibility requires validation [51,71,72].
Patient Safety
Each modality carries procedure-specific risks. Intermittent HD can precipitate intradialytic hypotension (10-15% of sessions) due to rapid fluid shifts, requires vascular access (infection risk), and mandates anticoagulation (bleeding risk). CRRT (HF/HDF) is gentler on hemodynamics but requires continuous anticoagulation and close monitoring in the ICU. Plasmapheresis risks include citrate-induced hypocalcemia, removal of clotting factors (coagulopathy), hypotension, and allergic reactions to replacement fluids (e.g., donor plasma). Hemoperfusion can cause transient thrombocytopenia and leukopenia (up to ~50% cell loss with uncoated columns), filter clotting, and hypoglycemia (adsorption of glucose) during prolonged use. Modern sorbents have reduced these events but still require anticoagulation. Nanofiber mesh systems, as currently conceptualized, would operate at low pressure without complex machinery. One study highlights a “wearable” PSA nanofiber patch needing no specialized equipment. In theory, passive absorption might cause less hemodynamic disturbance. However, no human safety data exist yet. Potential issues include thrombogenicity if fibers contact blood and saturation, leading to rapid clearance loss. Thus, patient safety for nanofiber devices remains theoretical [12,66,72,74].
Inflammatory and Immune Responses
Blood material contact inherently triggers immune responses. As noted, cellulose dialysis membranes provoke complement and neutrophil activation, whereas synthetic membranes are comparatively inert. HF/HDF filters are similar in composition, so their inflammatory activation is low. Plasmapheresis acutely reduces inflammatory mediators (CRP, cytokines) since it physically removes plasma, but the net immune effect is complex: one trial found no additional cytokine reduction from TPE beyond the natural decline in sepsis [69,70,73].
Hemoperfusion adsorbers remove cytokines and endotoxins, potentially dampening hyperinflammation. Devices like CytoSorb® report decreased IL-6 and TNF-α in septic patients. However, controlled data suggest that rapid endogenous cytokine kinetics often negate these interventions. Nanofiber meshes have not been evaluated for immune effects. Their materials (EVOH, zeolites) are non-immunogenic in concept. If used without plasma separation, blood cells would contact polymer fibers, so coating or flow design (e.g., plasma ultrafiltration followed by mesh adsorption) would be needed to minimize cell activation. In sum, conventional methods each provoke some inflammatory response (though modern materials minimize it), hemoadsorption aims to reduce cytokine storm, and nanofibers are too novel for clinical immunogenicity data [75-77].
Clinical Applications and Therapeutic Outcomes
Hemodialysis and HDF are standard for chronic kidney failure (CKD 5) and acute kidney injury (AKI). HD long-term improves symptoms and prolongs life in End Stage Renal Disease (ESRD), but 5-year survival on dialysis remains well below the general population. CRRT modalities (continuous venovenous HD) are widely used in ICU AKI, with similar survival to intermittent HD in trials; choice often depends on hemodynamic stability. Plasmapheresis is first-line for thrombotic microangiopathies like Thrombotic Thrombocytopenic Purpura (TTP), rapidly reversing disease and dramatically lowering mortality. It is also used in autoimmune neurological (GBS, myasthenia), renal (ANCA vasculitis), and transplant (antibody-mediated rejection) disorders. Hemoperfusion is established in poisoning management (drug overdoses, toxin removal), acute liver failure, and is emerging in sepsis and cytokine storm. For instance, Polymyxin B hemoperfusion is approved in some countries for septic shock with high endotoxin; CytoSorb® has been reported to improve survival parameters in case series of septic shock (though large RCT evidence is lacking). Nanofiber mesh devices are not yet in clinical use. Prototype “wearable” nanofiber filters have been tested only in vitro or in animal models, not in humans. Thus, clinical outcomes data exist only for traditional modalities. In practice, all extracorporeal therapies can improve acute biochemical abnormalities (e.g., reduce toxin levels) and stabilize patients, but none fully normalizes physiology or guarantees survival. Dialysis-dependent CKD patients still face high cardiovascular and infectious morbidity, and experimental adsorbents have yet to show outcome benefits in trials [25,78,79].
Implementation Complexities and Infrastructure
Hemodialysis requires extensive infrastructure: dialysis machines, water purification systems, dialysate production, and trained staff. Each session uses ~150+ L of purified water and takes several hours weekly. HDF uses dedicated ICU machines and sterile replacement/dialysate fluid; it runs continuously (24/7) and requires nursing in ICU settings. Plasmapheresis can utilize either centrifuge devices or large-pore dialysis membranes; it requires blood access and complex fluid management (plasma/albumin supply). Hemoperfusion devices are simpler cartridges of adsorbent packed into columns; these can be inserted into dialysis circuits, requiring only a blood pump [80-82].
Plasma-separation prior to sorbent use (to protect blood cells) adds complexity. Overall, hemoperfusion can be integrated into existing RRT setups. Nanofiber mesh filters promise minimal infrastructure: the zeolite–EVOH mesh was envisioned as a wearable arm patch, needing only blood flow through the mesh without dialysate or pumps. In one proposal, blood simply flows by capillary action through a stack of nanofiber mats. This could eliminate the need for large machines and continuous water/electricity. However, practical deployment would still require a blood pump or arterio-venous shunt, and sterilizable, disposable cartridges. In summary, conventional therapies leverage established hospital systems, whereas nanofiber-based approaches aim for simplicity and portability. Compatibility with existing devices is high for HD/HDF/TPE/HP (they share bloodlines and anticoagulation protocols); nanofiber devices would need new protocols and regulatory approval [83-85]. Performance metrics across conventional and nanofiber-based detox techniques are outlined in (Table 3).
|
Parameter |
Hemodialysis (HD) |
Hemodiafiltration (HDF) |
Plasmapheresis |
Hemoperfusion (HP) |
Nanofiber Mesh Technology |
References |
|
Clearance: Uremic Toxins |
Effective for small solutes; poor for PBUTs |
Better for middle molecules; limited PBUTs |
Removes PBUTs via plasma removal |
Adsorbs PBUTs (e.g., β₂-microglobulin) |
~ 67% creatinine removal; PBUT targeting possible |
[51,59,60,63]. |
|
Clearance: Inflammatory mediators / Cytokines |
Not effective |
Inconsistent clearance |
Removes some cytokines |
Adsorbs cytokines like IL-6, TNF-α |
Future selective cytokine binding is possible |
[59,60,61,72]. |
|
Clearance: Drugs / Toxins |
Works for small water-soluble drugs |
Similar to HD |
Excellent for protein-bound drugs |
Adsorbs a wide range of toxins |
Theoretical adaptability for drug/toxin removal |
[59,60,63,72]. |
|
Biocompatibility |
Good with synthetic membranes |
Similar to HD, the immune activation risk |
Generally good, but allergic reactions are possible |
Earlier toxicity resolved with coatings |
Non toxic, non thrombogenic |
[51,59,60,63,72]. |
|
Patient Safety |
Risks: hypotension, bleeding, infection. |
Gentler but needs anticoagulation |
Risks: bleeding, allergic reactions |
Risks: cytopenia, immune response |
Passive, pump-free; safe in preclinical use |
[12,51,60,63,72]. |
|
Immune response |
Minor complement and leukocyte activation |
Similar to HD |
Complex immune effects |
Reduces cytokines, but immune activation is possible |
No immune activation observed; potential for anti-inflammatory designs |
[51,59,60,63,72]. |
|
Clinical Applications |
ESRD, AKI |
ICU-based AKI |
TTP, autoimmune disorders |
Sepsis, liver failure, and drug overdose |
Experimental; wearable detox tested in vitro / animal models |
[12,51,60,63,72,77]. |
|
Implementation Complexity |
Needs dialysate, trained staff |
ICU setting; complex monitoring |
High logistics; costly |
Medium-high; adjunct to HD |
Simple, wearable, no external circuit |
[12,51,60,63,72,77]. |
Table 3: Comparative Analysis of Conventional and Nanofiber Mesh-Based Clinical Blood Detoxification Techniques
Nanofiber Innovations in Medical Applications
Zeolite Composite Nanofiber Mesh for Indoxyl Sulfate Adsorption
Sasaki et al. (2021) designed a zeolite composite nanofiber mesh that can adsorb indoxyl sulfate (IS), a toxin that is found to be linked to the development of chronic kidney disease. The study identified eight different types of zeolites and concluded that the zeolite with a pore size of 7 Å, H+ cations, and Si/Al ratio of 240 mol/mol adsorbed the most amount of IS. This optimal zeolite was then incorporated with nanofibers through the technique of electrospinning to form the composite mesh. The mesh also exhibited an IS adsorption capacity of 107 mug/g while zeolite, which was in a powdered form, showed an adsorption capacity of 208 mug/g, but in the mesh form, the same zeolite had an adsorption capacity of 386 mug/g. Further, the composite mesh improved the percentage of viable cells from 86% to 96%, which indicates the utility of the material in wearable blood purification systems [86].
Electrospun Cellulose Nanofiber as Affinity Membrane
Ma Z, Ramakrishna (2008) investigated how affinity filtration could be applied using cellulose nanofiber membranes. Electrospinning process in which a cellulose acetate (CA) solution was transformed to an electret fiber mesh and heat-treated, and the regenerated cellulose (RC) was produced from NaOH. In this work, the RC membrane surface was activated by the introduction of the affinity dye Cibacron Blue F3GA to improve the separation of biomolecules. The CB-functionalized membrane showed binding capacities of 13 for BSA and 4 for bilirubin. It also showed reusability and high filtration efficiency compared to some of the commercially available membranes, making the material suitable for biomolecule separations [87].
Enhanced Hemostatic Efficiency of Sonicated Chitosan Nanofiber Mats
Gu et al. (2013) constructed a chitosan nanofiber mat by using an electrospinning technique for the preparation of an efficient hemostatic material. To counteract water solubility, the best conditions for neutralization employing different kinds of alkalis were identified. To achieve this, the mat underwent ultrasonication that improved the porosity and structures of the mat, thereby raising porosity from 79.9%" to 97.2% and water absorption time from 110s to 9s. Sonicated chitosan mat has significantly higher efficiency on blood coagulation compared with that of Surgicel® and chitosan sponge, with an increase rate of 1.35 folds 3.41-fold, respectively. Moreover, fibroblast proliferation was: 1.4+/−0.2 on the sonicated mat compared to 1.0+/−0.2 on the [non-sonicated] mat, which could be useful in more complex applications of advanced wound healing [88].
Porous Nanofiber membrane for Endotoxin Removal
Wu et al. (2024) fabricated a porous nanofiber/microparticles (PNfMp) membrane based on the renal glomerular structure using an electrospray technique. The process employed a binary solvent of tetrahydrofuran (THF) and methanol to create highly porous structures of Poly(styrene-random-glycidyl methacrylate)(PSGM). It was able to get a very high surface area of about 986.6 m²/g, which was determined using the Brunauer-Emmett-Teller (BET). When the membrane surface was firmly attached to polymyxin B (PMB) on the epoxy groups, the adsorption ability of endotoxin was significantly enhanced to 3.1 EU/mg, while that of the non-porous membrane was 0.7 EU/mg. The authors described the use of this PMB-modified membrane filter for the purification of human plasma and achieved endotoxin reduction of approximately 99.7% at a flow rate of 1 mL/min [89].
Double-layered Nanofiber Filter for Urea & Ammonium Removal
Sasaki et al. (2024) proposed a double-layered nanofiber filter to alleviate the issues of urea and ammonium clearance in WAKs. Urease immobilized poly (ethylene-co-vinyl alcohol) (EVOH)/chitosan nanofiber demonstrated a considerable depollution rate of 690mg/g per hour. On the other hand, the sodium cobalt(II) hexacyanoferrate(II) (NaCoHCF) incorporated EVOH nanofiber has a maximum adsorption of ammonium up to 135.5mg/g. The comparison of the double-layered membrane indicated a good rate of urea breakdown, and ammonium decrease by 90% after cycling constantly. This is a great leap in the advancement of WAKs for usage in managing chronic kidney diseases [90].
Advanced Nanofiber Meshes for Improved Pelvic Organ Prolapse Treatment
Mukherjee et al. (2020) used endometrial mesenchymal stem/stromal cells (eMSCs) and bioengineered electrospun poly L-lactic acid-co-poly ε-caprolactone nanofibrous meshes (P nanomesh) for pelvic organ prolapse (POP) treatment. In one mouse model, increased integration with tissue, diminished acute inflammation, and increased score across 35 genes related TO ECM, angiogenesis, and immunomodulation were found with the bioengineered meshes in comparison with standard P nanomesh. This innovation serves to overcome some of the challenges posed by the traditional type of POP treatments [91].
Nanofiber-Enabled Blood Vessel Specification for Bone Repair
Zhai et al. (2021) studied the spatiotemporal characteristics of blood vessels in bone tissue regeneration with the help of nanofiber mesh constructs. Using the various imaging techniques, they characterized arterial-like (CD31+EMCN-) and venous-like (CD31+EMCN+) vessels, which displayed higher oxygen levels and flow rates in arterial-like vessels. It was also evidenced that donor osteoblast clusters are located around the newly established venous-like vessels, which interconnect with arterial-like vessels. This work also showed the difference in the distribution of vessel type in the regions with healing and non-healing defects, highlighting the importance of nanofiber-improved vascularization in the bone tissue regeneration [92].
Nanofiber Scaffolds for Acute Liver Failure Treatment
Liu et al. (2024) studied the application of nanofiber scaffolds in LTE as a possible substitute for transplantation for ALF, which has a more than 40% mortality rate. These scaffolds resemble the structure of the ground substance that forms the basis of an ECM and encourages the growth of cells and the healing of tissues. The review focuses on recent developments in fabricating scaffolds, the application of scaffolds in controlling liver cell functions, and the challenge of linking seeding cells together with growth factors. It also describes the present issues and the future direction with regard to the perspective scaffold design for the treatment of ALF [93].
EGCG-Loaded PLGA Nanofiber Membranes for Adhesion Prevention
Shin et al. (2014) fabricated biodegradable nanofiber membranes composed of PLGA containing EGCG for the prevention of postsurgical adhesions. The membranes, which had fibers with an average diameter of 300-500 nm, demonstrated a controlled release of EGCG over 28 days due to diffusion and the degradation of PLGA. EGCG improved the antioxidant characteristics and biocompatibility of membranes, reducing the formation of HO and increasing the clotting time. The in vivo studies employing the rat models showed that the EGCG-enriched PLGA membranes effectively mitigated the formation of tissue adhesions, as indicated in comparable outcomes with the commercial antiadhesion barrier products. These results point to the possibility of using PLGA nanofibers incorporating EGCG in surgical applications as an effective modality in enhancing the healing rate and preventing the formation of adhesions [94].
Nanofiber-Based Endothelialization for Blood-Compatible Interfaces
Weidenbacher et al. (2019) designed a nanofiber-based interface for endothelialization on elastic silicone material used in ventricular assist devices (VADs). They developed an elastic polyurethane and poly (vinylidene fluoride-co-hexafluoropropylene) membrane that is embedded in silicone that minimizes deformation and increases antithrombotic characteristics. For example, to create an environment in a pulsatile flow bioreactor that mimics the conditions of in vivo conditions, this material was characterized in maintaining stable endothelial cell formation. This approach holds promise for the development of blood-compatible materials for the next-generation VADs [95].
Future Directions
The discussion would be incomplete without emphasizing the adaptable nature of nanofiber mesh technology. This also includes new materials like nanoparticles, biomolecules, or responsive polymers for better designing of these meshes to selectively bind a broad range of toxins, such as heavy metals, synthetic drugs, and metabolic by-products [96]. Further investigations into optimizing pore size and surface area for increasing binding efficiency for various toxins could guarantee faster and more selective detoxification procedures. These advances can very much expand the therapeutic horizon for nanofiber meshes in clinical practice [97-99].
Developing the Nanofiber meshes to meet the requirements of patient specificity must be focused along with the adaptability of meshes in different fields of study. These would include designing meshes based on individual patient profiles, taking into account the type of toxins, nature of the body, and treatment durations. Also, the practices using 3D printing or computational modeling would obtain this capability. These would be complemented by the inclusion of antimicrobial agents into the mesh to avoid secondary infections during detoxification, and those are often concerning in critically ill or immunocompromised patients. Together, these would guarantee advances in positioning nanofiber meshes as integral elements within precision medicine [100,101]. There are other exciting avenues to explore around synergistic opportunities from nanofiber mesh technology and real-time monitoring systems. Embedding such sensors within the mesh effectively allows for continuous tracking of the toxin levels throughout the detoxification process and points out such essential data to the clinicians. This would certainly bring up the adaptability of the detoxification protocol in various therapeutic studies, resulting in some positive outcomes. The additional developments toward reusable or self-cleaning nanofiber meshes should address cost evaluation and increase the opportunities for widespread clinical use of nanofiber mesh technology. These forward-looking strategies articulate with significant goal for enhancing the efficacy, safety, and sustainable detoxification therapies [102,103].
Conclusion
The transition from conventional blood detoxification techniques to nanofiber mesh-based systems reflects a significant leap in therapeutic innovation. Nanofiber meshes demonstrate a combination of structural precision, customizable surface chemistry, and excellent biocompatibility, making them well-suited for clinical applications. Their selective adsorption mechanisms allow for efficient removal of toxins—including protein-bound and hydrophobic compounds—while minimizing harm to essential blood components. Furthermore, advancements in fabrication processes such as electrospinning have enabled scalable production of functional meshes with high reproducibility. Compared to traditional modalities, nanofiber systems offer the potential for portable, cost-effective, and patient-friendly solutions. However, despite encouraging laboratory results, large-scale clinical trials and long-term safety evaluations remain essential before these technologies can be adopted into routine medical practice. With continued interdisciplinary research, nanofiber mesh technology may redefine standards in blood purification, offering improved patient outcomes and reducing the burden on critical care systems.
Ethical Statement
This article is a narrative review that does not involve any new studies with human participants or animals performed by any of the authors. Therefore, ethical approval was not required for this work.
Consent to Participate
This article is based solely on previously published literature. No human participants were involved, and no personal data were collected. Therefore, informed consent to participate is not applicable.
Funding
This review article did not receive any funding from an institution or organization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Gnanendra Sai Kumar Nareboina: Conceptualization, Data curation, Methodology, Resources, Writing – original draft, Writing – review & editing, Visualization, Supervison.
Poojitha Bandaru: Resources, Data curation, Investigation, Visualization, Writing – original draft.
Uma Bekkam: Resources, Investigation, Formal Analysis, Writing – original draft.
Reena Varshitha Saripalli: Formal Analysis, Software, Visualization.
Keerthi Vallapureddy: Resources, Visualization, Data curation.
Satya Jagadeesh Botta: Investigation, Validation, Software.
Girish Kombathula: Validation, Formal Analysis, Investigation.
Pavan Kumar Pothanapalli: Formal Analysis, Investigation, Validation.
- Camporota L, Corno E, Menaldo E, Smith J, Lei K, Beale R, Wyncoll D. (2008). Filter survival time and requirement of blood products in patients with severe sepsis receiving drotrecogin alfa (activated) and requiring renal replacement therapy. Critical Care. 12(6): 163.
- Rasouli R, Barhoum A, Bechelany M, Dufresne A. (2019). Nanofibers for biomedical and healthcare applications. Macromolecular bioscience. 19(2): 1800256.
- Stuard S, Ridel C, Cioffi M, Trost-Rupnik A, Gurevich K, Bojic M, Karibayev Y, Mohebbi N, Marcinkowski W, Kupres V, Maslovaric J. (2024). Hemodialysis procedures for stable incident and prevalent patients optimize hemodynamic stability, dialysis dose, electrolytes, and fluid balance. Journal of Clinical Medicine. 13(11): 3211.
- Bhardwaj N, Kundu SC. (2010). Electrospinning: A fascinating fiber fabrication technique. Biotechnology advances. 28(3): 325-347.
- Lang T, Zawada AM, Theis L, Braun J, Ottillinger B, Kopperschmidt P, Gagel A, Kotanko P, Stauss-Grabo M, Kennedy JP, Canaud B. (2023). Hemodiafiltration: technical and medical insights. Bioengineering. 10(2): 145.
- Legallais C, Kim D, Mihaila SM, Mihajlovic M, Figliuzzi M, Bonandrini B, Salerno S, Yousef Yengej FA, Rookmaaker MB, Sanchez Romero N, Sainz‐Arnal P. (2018). Bioengineering organs for blood detoxification. Advanced healthcare materials. 7(21): 1800430.
- Connelly‐Smith L, Alquist CR, Aqui NA, Hofmann JC, Klingel R, Onwuemene OA, Patriquin CJ, Pham HP, Sanchez AP, Schneiderman J, Witt V. (2023). Guidelines on the use of therapeutic apheresis in clinical practice–evidence‐based approach from the Writing Committee of the American Society for Apheresis: the Ninth Special Issue. Journal of Clinical Apheresis. 38(2): 77-278.
- Nizar OA, Rai P, Rao SN, Shenoy MP. (2017). Plasmapheresis: A retrospective audit of procedures from a tertiary care center in Southern India. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine. 21(12): 857-860.
- Ricci Z, Romagnoli S, Reis T, Bellomo R, Ronco C. (2022). Hemoperfusion in the intensive care unit. Intensive care medicine. 48(10): 1397-1408.
- Howell BA, Chauhan A. (2010). Current and emerging detoxification therapies for critical care. Materials. 3(4): 2483-2505.
- Bouchard J, Khosla N, Mehta RL. (2008). Emerging therapies for extracorporeal support. Nephron Physiology. 109(4): 85-91.
- Ronco C, Bellomo R. (2022). Hemoperfusion: technical aspects and state of the art. Critical care. 26(1): 135.
- Qu X, Gou M, Zaidan J, Zhang K, Chen S. (2014). Challenges and opportunities in developing nanoparticles for detoxification. Nanomedicine. 9(16): 2437-2439.
- Kernchen RJ. (2010). Enzyme stabilization in nanostructured materials, for use in organophosphorus nerve agent detoxification and prophylaxis. InBiodefence: advanced materials and methods for health protection. Dordrecht: Springer Netherlands. 135-145.
- Pashirova TN, Bogdanov A, Masson P. (2021). Therapeutic nanoreactors for detoxification of xenobiotics: Concepts, challenges and biotechnological trends with special emphasis to organophosphate bioscavenging. Chemico-Biological Interactions. 346: 109577.
- Zhang F, Jacobs AI, Woodall M, Hailes HC, Uchegbu IF, Fernandez-Reyes D, Smith CM, Dziemidowicz K, Williams GR. (2024). A one-step method for generating antimicrobial nanofibre meshes via coaxial electrospinning. Materials Advances. 5(13): 5561-5571.
- Zhou J, Li X, Zhang Z, Hou T, Xu J, Wang Y, Ye H, Yang B. (2024). Bio-based and bio-degradable nanofiber materials: A sustainable platform for energy, environmental, and biomedical applications. Chemical Engineering Journal. 491: 152105.
- Ng LF, Yahya MY, Hamzah SM, San Khoo P. (2023). Preparation and Characterization of the Electrospun Nanofiber Meshes. InElectrospun Nanofibres. CRC Press. 109-127.
- Qi J, Zhu C, Hu J, Ran S, Ahmad T, Ullah S, Chen L, Chen Y, Xia Y, Sun X, Li C. (2024). B/N modified carbon nanofiber mesh covered with layered double hydroxide for large current overall water splitting. Fuel. 371: 131983.
- Adil HI, Thalji MR, Yasin SA, Saeed IA, Assiri MA, Chong KF, Ali GA. (2022). Metal–organic frameworks (MOFs) based nanofiber architectures for the removal of heavy metal ions. Rsc Advances. 12(3): 1433-1450.
- Ronco C, Crepaldi C, Brendolan A, Bragantini L, d'Intini V, Inguaggiato P, Bonello M, Krause B, Deppisch R, Goehl H, Scabardi A. (2003). Evolution of synthetic membranes for blood purification: the case of the Polyflux family. Nephrology Dialysis Transplantation. 18(7): vii10-20.
- Barhoum A, Pal K, Rahier H, Uludag H, Kim IS, Bechelany M. (2019). Nanofibers as new-generation materials: From spinning and nano-spinning fabrication techniques to emerging applications. Applied Materials Today. 17(15): 1-35.
- Yan B, Zhang Y, Li Z, Zhou P, Mao Y. (2022). Electrospun nanofibrous membrane for biomedical application. SN Applied Sciences. 4(6): 172.
- Sasaki M, Hirata R, Konagai A, Ebara M. (2024). Electrospun EVOH/AST-120 hybrid nanofiber membranes for removal of indoxyl sulfate from blood. RSC advances. 14(36): 26596-26603.
- Namekawa K, Schreiber MT, Aoyagi T, Ebara M. (2014). Fabrication of zeolite–polymer composite nanofibers for removal of uremic toxins from kidney failure patients. Biomaterials Science. 2(5): 674-679.
- Fu X, Shi M, Chen D, Zhao X, Jiang T, Zhao R. (2024). Theory-Driven Tailoring of the Microenvironment of Quaternary Ammonium Binding Sites on Electrospun Nanofibers for Efficient Bilirubin Removal in Hemoperfusion. Polymers. 16(11): 1599.
- Li W, Chao S, Li Y, Bai F, Teng Y, Li X, Li L, Wang C. (2022). Dual-layered composite nanofiber membrane with Cu-BTC-modified electrospun nanofibers and biopolymeric nanofibers for the removal of uremic toxins and its application in hemodialysis. Journal of Membrane Science. 642(32): 119964.
- Zhao R, Li Y, Li X, Li Y, Sun B, Chao S, Wang C. (2018). Facile hydrothermal synthesis of branched polyethylenimine grafted electrospun polyacrylonitrile fiber membrane as a highly efficient and reusable bilirubin adsorbent in hemoperfusion. Journal of colloid and interface science. 514: 675-685.
- Kuddushi M, Pawar AS, Ghaffari Sharaf M, Unsworth LD, Zhang X. (2025). Evaluating Electrospun Polycaprolactone Fibers for Blood‐Contacting Applications. Biopolymers. 116(2): 23656.
- Ma Z, Kotaki M, Ramakrishna S. (2005). Electrospun cellulose nanofiber as affinity membrane. Journal of membrane science. 265(1-2): 115-123.
- Liu M, Zhang W, Zhang P, Yuan L, Li H, Zhan J, Zhao W, Mei X. (2023). Enhanced hemocompatibility of lysine grafted polyacrylonitrile electrospun nanofiber membranes as a potential bilirubin adsorption in hemoperfusion. Journal of Polymers and the Environment. 31(4): 1553-1567.
- Yuan Z, Li Y, Zhao D, Zhang K, Wang F, Wang C, Wen Y. (2018). High efficiency 3D nanofiber sponge for bilirubin removal used in hemoperfusion. Colloids and Surfaces B: Biointerfaces. 172: 161-169.
- Li X, Wei F, Li Y, Qi F, Wang J, Yao Z, Zhang L, Zhang B. (2024). Fabricating polyacrylonitrile-polyethyleneimine nanofiber membranes with exceptional hemocompatibility and efficient bilirubin removal for hemoperfusion. Reactive and Functional Polymers. 194(4): 105793.
- Zhang L, Liu G, Xia Q, Deng L. (2024). Research progress on blood compatibility of hemoperfusion adsorbent materials. Frontiers in Bioengineering and Biotechnology. 12:1456694.
- Mahdipour E, Mequanint K. (2022). Films, gels and electrospun fibers from serum albumin globular protein for medical device coating, biomolecule delivery and regenerative engineering. Pharmaceutics. 14(11): 2306.
- Yamazoe H, Tanabe T. (2010). Drug-carrying albumin film for blood-contacting biomaterials. Journal of Biomaterials Science, Polymer Edition. 21(5): 647-657.
- Kottke-Marchant K, Anderson JM, Umemura Y, Marchant RE. (1989). Effect of albumin coating on the in vitro blood compatibility of Dacron® arterial prostheses. Biomaterials. 10(3):147-155.
- Marois Y, Chakfé N, Guidoin R, Duhamel RC, Roy R, Marois M, King MW, Douville Y. (1996). An albumin-coated polyester arterial graft: in vivo assessment of biocompatibility and healing characteristics. Biomaterials. 17(1): 3-14.
- Najafi M, Frey MW. (2020). Electrospun nanofibers for chemical separation. Nanomaterials. 10(5): 982.
- Gao C, Zhang Q, Yang Y, Li Y, Lin W. (2022). Recent trends in therapeutic application of engineered blood purification materials for kidney disease. Biomaterials research. 26(1): 5.
- Zhang Y, Lim CT, Ramakrishna S, Huang ZM. (2005). Recent development of polymer nanofibers for biomedical and biotechnological applications. Journal of materials science: materials in medicine. 16(10): 933-946.
- Ramakrishna S, Fujihara K, Teo WE, Yong T, Ma Z, Ramaseshan R. (2006). Electrospun nanofibers: solving global issues. Materials today. 9(3): 40-50.
- Wang X, Yu J, Sun G, Ding B. (2016). Electrospun nanofibrous materials: a versatile medium for effective oil/water separation. Materials today. 19(7): 403-414.
- Huang Y, Yuan Z, Zhao D, Wang F, Zhang K, Li Y, Wen Y, Wang C. (2019). Polymyxin B immobilized nanofiber sponge for endotoxin adsorption. European Polymer Journal. 110:69-75.
- Wang X, Hsiao BS. (2016). Electrospun nanofiber membranes. Current opinion in chemical engineering. 12: 62-81.
- Strazielle N, Khuth ST, Ghersi-Egea JF. (2004). Detoxification systems, passive and specific transport for drugs at the blood–CSF barrier in normal and pathological situations. Advanced drug delivery reviews. 56(12): 1717-1740.
- Shen Y, Shen Y, Bi X, Shen A, Wang Y, Ding F. (2024). Application of nanoparticles as novel adsorbents in blood purification strategies. Blood Purification. 53(9): 743-754.
- Wang Y, Wei R, Zhao W, Zhao C. (2023). Bilirubin removal by polymeric adsorbents for hyperbilirubinemia therapy. Macromolecular Bioscience. 23(5): 2200567.
- Wang L, Han K, Jiang X, Mao C, Li X, Zhou M. (2024). Recent advances in blood toxin removal technology. Sustainable Materials and Technologies. 39(1): 00828.
- Araujo JC, Fangueiro R, Ferreira DP. (2021). Protective multifunctional fibrous systems based on natural fibers and metal oxide nanoparticles. Polymers. 13(16): 2654.
- Tsuge M, Takahashi K, Kurimoto R, Fulati A, Uto K, Kikuchi A, Ebara M. (2019). Fabrication of water absorbing nanofiber meshes toward an efficient removal of excess water from kidney failure patients. Fibers. 7(5): 39.
- Nayak R, Padhye R, Kyratzis IL, Truong YB, Arnold L. (2012). Recent advances in nanofibre fabrication techniques. Textile Research Journal. 82(2): 129-147.
- Wang Y, Wakisaka M. (2014). Nanofiber fabrication techniques and its applicability to chitosan. Progress in Chemistry. 26(11): 1821-1831.
- Park S, Park K, Yoon H, Son J, Min T, Kim G. (2007). Apparatus for preparing electrospun nanofibers: designing an electrospinning process for nanofiber fabrication. Polymer International. 56(11): 1361-1366.
- Abdel-Hady F, Alzahrany A, Hamed M. (2011). Experimental validation of upward electrospinning process. International Scholarly Research Notices. 2011(1): 851317.
- Alghoraibi I, Alomari S. (2018). Different methods for nanofiber design and fabrication. InHandbook of nanofibers. Springer, Cham. 1-46.
- Islam MS, Ang BC, Andriyana A, Afifi AM. (2019). A review on fabrication of nanofibers via electrospinning and their applications. SN Applied Sciences. 1(10): 1248.
- Tang L, Jia M, He M, Liu Q, Lin Y, Yi Y, Liu X, Liu X, Tang Y, Gu J. (2024). Fabrication, applications, and prospects for poly (p‐phenylene benzobisoxazole) nanofibers. SusMat. 4(6): 245.
- Yamamoto S, Kazama JJ, Wakamatsu T, Takahashi Y, Kaneko Y, Goto S, Narita I. (2016). Removal of uremic toxins by renal replacement therapies: a review of current progress and future perspectives. Renal Replacement Therapy. 2(1): 43.
- Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. (2006). Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrology Dialysis Transplantation. 21(5): 1300-1304.
- Tang WW, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. (2013). Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine. 368(17): 1575-1584.
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, DuGar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. nature. 472(7341): 57-63.
- Abe T, Uchita K, Orita H, Kamimura M, Oda M, Hasegawa H, Kobata H, Fukunishi M, Shimazaki M, Abe T, Akizawa T. (2003). Effect of β2-microglobulin adsorption column on dialysis-related amyloidosis. Kidney international. 64(4): 1522-1528.
- Gejyo F, Kawaguchi Y, Hara S, Nakazawa R, Azuma N, Ogawa H, Koda Y, Suzuki M, Kaneda H, Kishimoto H, Oda M. (2004). Arresting dialysis‐related amyloidosis: a prospective multicenter controlled trial of direct hemoperfusion with a β2‐microglobulin adsorption column. Artificial organs. 28(4): 371-380.
- Lekawanvijit S, Kompa AR, Wang BH, Kelly DJ, Krum H. (2012). Cardiorenal syndrome: the emerging role of protein-bound uremic toxins. Circulation Research. 111(11): 1470-1483.
- Ahmed S, Kaplan A. (2020). Therapeutic plasma exchange using membrane plasma separation. Clinical Journal of the American Society of Nephrology. 15(9): 1364-1370.
- Lumlertgul N, Hall A, Camporota L, Crichton S, Ostermann M. (2021). Clearance of inflammatory cytokines in patients with septic acute kidney injury during renal replacement therapy using the EMiC2 filter (Clic-AKI study). Critical Care. 25(1): 39.
- Atan R, Crosbie D, Bellomo R. (2012). Techniques of extracorporeal cytokine removal: a systematic review of the literature. Blood purification. 33(1-3): 88-100.
- Mehta Y, Paul R, Ansari AS, Banerjee T, Gunaydin S, Nassiri AA, Pappalardo F, Premužić V, Sathe P, Singh V, Vela ER. (2023). Extracorporeal blood purification strategies in sepsis and septic shock: An insight into recent advancements. World journal of critical care medicine. 12(2): 71-88.
- Sauer A, Stahl K, Seeliger B, Wendel-Garcia PD, Lehmann F, Schmidt JJ, Schmidt BM, Welte T, Peukert K, Wild L, Putensen C. (2025). The effect of therapeutic plasma exchange on the inflammatory response in septic shock: a secondary analysis of the EXCHANGE-1 trial. Intensive Care Medicine Experimental. 13(1)
- Cervantes CE, Bloch EM, Sperati CJ. (2023). Therapeutic plasma exchange: core curriculum 2023. American Journal of Kidney Diseases. 81(4): 475-492.
- Gutierrez A, Alvestrand A, Bergström J, Beving H, Lantz B, Henderson LW. (1994). Biocompatibility of hemodialysis membranes: a study in healthy subjects. Blood purification. 12(2): 95-105.
- Li A, Zhang J, Wang A. (2005). Synthesis, characterization and water absorbency properties of poly (acrylic acid)/sodium humate superabsorbent composite. Polymers for advanced technologies. 16(9): 675-680.
- Chen Y, Tan HM. (2006). Crosslinked carboxymethylchitosan-g-poly (acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydrate Research. 341(7): 887-896.
- Soldatkin OO, Soy E, Errachid A, Jaffrezic-Renault N, Akata B, Soldatkin AP, Dzyadevych SV. (2011). Influence of composition of zeolite/enzyme nanobiocomposites on analytical characteristics of urea biosensor based on ion-selective field-effect transistors. Sensor Letters. 9(6):2320-2326.
- Bergé-Lefranc D, Vagner C, Calaf R, Pizzala H, Denoyel R, Brunet P, Ghobarkar H, Schäf O. (2012). In vitro elimination of protein bound uremic toxin p-cresol by MFI-type zeolites. Microporous and mesoporous materials. 153: 288-293.
- Webster AC, Nagler EV, Morton RL, Masson P. (2017). Chronic kidney disease. The lancet. 389(10075): 1238-1252.
- Tonelli M, Wiebe N, Knoll G, Bello A, Browne S, Jadhav D, Klarenbach S, Gill J. (2011). Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. American journal of transplantation. 11(10): 2093-2109.
- Nordio M, Limido A, Maggiore U, Nichelatti M, Postorino M, Quintaliani G, Registry T. (2012). Survival in patients treated by long-term dialysis compared with the general population. American journal of kidney diseases. 59(6): 819-828.
- Harris A, Cooper BA, Li JJ, Bulfone L, Branley P, Collins JF, Craig JC, Fraenkel MB, Johnson DW, Kesselhut J, Luxton G. (2011). Cost-effectiveness of initiating dialysis early: a randomized controlled trial. American journal of kidney diseases. 57(5): 707-715.
- Baboolal K, McEwan P, Sondhi S, Spiewanowski P, Wechowski J, Wilson K. (2008). The cost of renal dialysis in a UK setting—a multicentre study. Nephrology Dialysis Transplantation. 23(6):1982-1989.
- Young BA, Chan C, Blagg C, Lockridge R, Golper T, Finkelstein F, Shaffer R, Mehrotra R. (2012). ASN Dialysis Advisory Group. How to overcome barriers and establish a successful home HD program. Clinical journal of the American Society of Nephrology. 7(12): 2023-2032.
- See EJ, Alrukhaimi M, Ashuntantang GE, Bello AK, Bellorin-Font E, Gharbi MB, Braam B, Feehally J, Harris DC, Jha V, Jindal K. (2018). Global coverage of health information systems for kidney disease: availability, challenges, and opportunities for development. Kidney International Supplements. 8(2): 74-81.
- Takai R, Kurimoto R, Nakagawa Y, Kotsuchibashi Y, Namekawa K, Ebara M. (2016). Towards a rational design of zeolite-polymer composite nanofibers for efficient adsorption of creatinine. Journal of Nanomaterials. 2016(1): 1-7.
- Rajala JW, Shin HU, Lolla D, Chase GG. (2015). Core–shell electrospun hollow aluminum oxide ceramic fibers. Fibers. 3(4): 450
- Sasaki M, Liu Y, Ebara M. (2021). Zeolite composite nanofiber mesh for indoxyl sulfate adsorption toward wearable blood purification devices. Fibers. 9(6): 37.
- Ma Z, Ramakrishna S. (2008). Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein A/G for IgG purification. Journal of Membrane Science. 319(1-2): 23-8.
- Gu BK, Park SJ, Kim MS, Kang CM, Kim JI, Kim CH. (2013). Fabrication of sonicated chitosan nanofiber mat with enlarged porosity for use as hemostatic materials. Carbohydrate Polymers. 97(1): 65-73.
- Wu TC, Cheng CC, Lu CH, Chen JK. (2024). Porous nanofiber/microparticles-structured membrane of polymyxin B modified poly (styrene-random-glycidyl methacrylate) by electrospray for endotoxin removal in blood purification. Journal of Membrane Science. 710(22): 123142.
- Sasaki M, Szabo L, Uto K, Ebara M. (2024). Double-Layered Electrospun Nanofiber Filter for the Simultaneous Removal of Urea and Ammonium from Blood. ACS Applied Materials & Interfaces. 16(49): 67399-67410.
- Mukherjee S, Darzi S, Paul K, Cousins FL, Werkmeister JA, Gargett CE. (2020). Electrospun nanofiber meshes with endometrial MSCs modulate foreign body response by increased angiogenesis, matrix synthesis, and anti-inflammatory gene expression in mice: implication in pelvic floor. Frontiers in Pharmacology. 11: 353.
- Zhai Y, Schilling K, Wang T, El Khatib M, Vinogradov S, Brown EB, Zhang X. (2021). Spatiotemporal blood vessel specification at the osteogenesis and angiogenesis interface of biomimetic nanofiber-enabled bone tissue engineering. Biomaterials. 276: 121041.
- Liu X, Yao X, OuYang Q, Oliveira AL, Yan L, Zhang Y. (2024). Nanofiber scaffold-based tissue engineering for the treatment of acute liver failure. Advanced Fiber Materials. 6(3): 686-712.
- Shin YC, Yang WJ, Lee JH, Oh JW, Kim TW, Park JC, Hyon SH, Han DW. (2014). PLGA nanofiber membr
Download Provisional PDF Here
PDF




p (1).png)




.png)




.png)
