Research Article
Pattern of Use of Antibacterial Agents at a Private Hospital in Yenagoa, Bayelsa State, South-South, Nigeria
- Dr. Owonaro A Peter
Corresponding author: Dr. Owonaro A Peter
Volume: 1
Issue: 3
Article Information
Article Type : Research Article
Citation : Peter A Owonaro, Ebena Micah, Sounyo Adebukola, Daughter E A Owonaro, Seiyefa Brisibe, Adam Solon. Pattern of Use of Antibacterial Agents at a Private Hospital in Yenagoa, Bayelsa State, South-South, Nigeria. Journal of Medical and Clinical Case Reports 1(3). https://doi.org/10.61615/JMCCR/2024/MAY027140525
Copyright: © 2024 Owonaro A Peter. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
DOI: https://doi.org/10.61615/JMCCR/2024/MAY027140525
Publication History
Received Date
09 May ,2024
Accepted Date
21 May ,2024
Published Date
25 May ,2024
Antibacterial agents, commonly known as antibiotics, are among the most widely prescribed and utilized medications in healthcare settings worldwide. However, the widespread and often indiscriminate use of antibiotics has led to concerning patterns of use, contributing to the emergence and spread of antimicrobial resistance (AMR), a significant global public health threat. This study aimed to investigate the pattern of use of antibacterial agents in a private hospital in Yenagoa, Bayelsa State, Nigeria. The objectives of the study were to determine the most frequently used class of antibiotics, identify the percentage of antibiotics prescribed by route of administration, and identify the percentage of antibiotics prescribed as generics. The study employed a retrospective approach, utilizing a carefully designed data collection form to assess the pattern of antibiotic use in the selected private hospital. Patient folders with prescriptions containing antibiotics between January and December 2019 were reviewed. Convenience sampling was used to select the study facility, time frame, and folders. Descriptive statistics were employed for data analysis using Microsoft Excel 2021. The demographic data revealed a slightly higher prescription rate for female patients (52.59%) and a significant portion of prescriptions for outpatients (64.31%). The age group with the highest prescription rate was 26-30 years (13.18%). Penicillins emerged as the most frequently prescribed class of antibiotics (38.19%), followed by Nitroimidazoles (21.43%) and Cephalosporins (15.08%). Oral administration was predominant (70%), and a substantial majority of prescriptions were for generic antibiotics (88.31%). The pattern of antibacterial use in this private hospital reflects a pragmatic approach focused on widely used antibiotic classes, convenient administration routes, and cost-effective generic options. The findings suggest a reliance on Penicillins, Nitroimidazoles, and Cephalosporins for treating infections, with a preference for oral administration due to its convenience and suitability for outpatient treatment. The high prescription rate for generic antibiotics highlights a focus on cost-effective treatment options without compromising therapeutic efficacy. The study provides insights into the pattern of antibacterial agent use in a private hospital in Yenagoa, Bayelsa State, Nigeria, identifying the most commonly prescribed antibiotic classes, routes of administration, and the predominance of generic prescriptions. Future research could explore the clinical outcomes associated with these patterns to ensure optimal patient care and adherence to antimicrobial stewardship principles. Additionally, implementing comprehensive antibiotic stewardship programs and continuous monitoring of antibiotic use patterns in private healthcare facilities could contribute to combating the global threat of antimicrobial resistance.
►Pattern of Use of Antibacterial Agents at a Private Hospital in Yenagoa, Bayelsa State, South-South, Nigeria
Peter A Owonaro1*, Ebena Micah1, Sounyo Adebukola1, Daughter E A Owonaro1, Seiyefa Brisibe1, Adam Solon1,
1Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmacy, Niger Delta University, Nigeria.
Keywords: Antibiotics, Penicillins, Nitroimidazoles, and Cephalosporins.
Introduction
Antibacterial agents, commonly known as antibiotics, are among the most widely prescribed and utilized medications in healthcare settings worldwide [35]. Their introduction has revolutionized the treatment of bacterial infections, significantly reducing morbidity and mortality rates associated with infectious diseases [13]. However, the widespread and often indiscriminate use of antibiotics has led to concerning patterns of use, contributing to the emergence and spread of antimicrobial resistance (AMR), a significant global public health threat [38]. The inappropriate and excessive use of antibiotics is a complex issue influenced by various factors, including patient-related, healthcare provider-related, and healthcare system-related factors [27,23,30]. These factors often interact and contribute to varying patterns of antibiotic use across different regions, healthcare settings, and patient populations [20]. In Nigeria, where antibiotics are readily available over the counter, and self-medication is common, the potential for inappropriate antibiotic use is heightened [26,15]. Furthermore, limited data on antibiotic use patterns in healthcare facilities, particularly in private hospitals, hinders the development and implementation of effective strategies to promote responsible antibiotic prescribing and combat AMR [14]. The rise of AMR poses significant challenges for healthcare systems globally, including increased healthcare costs, prolonged hospital stays, and limited treatment options for infections [11,28]. Additionally, AMR threatens the effectiveness of various medical procedures that rely on antibiotics to prevent and treat infections, such as organ transplantation, cancer chemotherapy, and major surgeries [31].
Private hospitals operating within a market-driven healthcare system face unique challenges and opportunities regarding antibiotic use and stewardship [25]. Financial incentives, profit motives, and patient satisfaction concerns may influence antibiotic prescribing decisions, potentially leading to overuse and misuse [27, 11]. Additionally, many private hospitals, particularly those with limited resources, may lack robust antibiotic stewardship programs (ASPs) and comprehensive surveillance systems for monitoring antibiotic use patterns and resistance trends [29, 2]. However, private hospitals also have opportunities to implement tailored ASPs, prioritize effective infection prevention and control measures, invest in advanced diagnostic tools, and provide continuous professional development for healthcare providers [2, 37, 9, 6]. By capitalizing on these opportunities, private hospitals can optimize antibiotic use, improve patient outcomes, and contribute to the global effort to combat AMR. In Yenagoa, the capital city of Bayelsa State in Nigeria, private hospitals play a significant role in providing healthcare services to the local population. However, there is a paucity of data on the pattern of antibacterial agent use in these facilities, which hinders the development and implementation of targeted interventions to promote responsible antibiotic prescribing and address AMR.
Objectives
- To determine the most frequently used class of antibiotics.
- To identify the percentage of antibiotics prescribed by route of administration.
- To identify the percentage of antibiotics prescribed by generics.
Method
Study Area
Family Care Hospital is a private health institution located in Azikoro town, in the Yenagoa local government area of Bayelsa state. It plays a vital role in providing healthcare services to the local population, serving as a hub for primary and secondary medical care, and addressing the healthcare needs of the locale.
Study Setting
This research was conducted at Family Care Hospital in Opolo town, in Yenegoa Local Government Area of Bayelsa State. The study focused on patient folders and prescriptions containing antibiotics between January to December 2019. This study spanned one month, from 24th January to 23 February 2024. Only folders that met the inclusion criteria were reviewed for the study.
Study Design
This study was a retrospective approach, using a carefully designed data collection form to assess the pattern of antibiotic use in this selected hospital. Only patient folders with prescriptions containing antibiotics were included, while folders with prescriptions that did not include antibiotics were excluded from the study.
Sampling
A convenience sampling technique was adopted in selecting the study facility as well as the time frame and folders to survey.
Data Collection
Retrospective review of patient records to collect data on antibiotic prescriptions, including the type and class of antibiotic as well as route of administration and indication. This data was analyzed to identify patterns and evaluate the appropriateness of antibiotic prescribing.
Statistical Analysis
Quantitative data was analyzed using descriptive statistics. Descriptive statistics, such as frequencies and percentages, were used to summarize the patterns of antibiotic use. All these were done using Microsoft Excel 2021 and the result was presented in tables and charts.
Ethical Approval
Ethical clearance was obtained from the Bayelsa State Health Research Ethics Committee with approval number BSHREC/Vol.1/24/02/16. And prior approval was obtained from the facility (hospital) before the commencement of data collection. The clearance certificate can be found in the appendix.
Results
Demography
The demographic data shows that the majority of the prescriptions were to female patients (52.29%) while 13.18% of them fell within ages 26 – 30, closely followed by those within ages 16 – 20 (12.48%). Furthermore, a chunk of the patients were out-patients representing 64.31%. Further demographic details can be found in Table 1 below.
Table 1: Summary of Demographics for 2019 (January to December) (N =1586)
|
Variables |
Frequency (%) |
|
GENDER |
|
|
Male |
742 (46.78%) |
|
Female |
834 (52.59%) |
|
Missing |
10 (0.63%) |
|
AGE |
|
|
0 – 5 |
104 (6.56%) |
|
6 – 10 |
138 (8.70%) |
|
11 – 15 |
45 (2.84%) |
|
16 – 20 |
198 (12.48%) |
|
21 – 25 |
174 (10.97%) |
|
26 – 30 |
209 (13.18%) |
|
31 – 35 |
142 (8.95%) |
|
36 – 40 |
180 (11.35%) |
|
41 – 45 |
136 (8.58%) |
|
46 – 50 |
73 (4.60%) |
|
51 – 55 |
71 (4.48%) |
|
56 – 60 |
68 (4.29%) |
|
61 – 65 |
7 (0.44%) |
|
66 – 70 |
15 (0.95%) |
|
>70 |
0 (0.00%) |
|
MISSING |
26 (1.64%) |
|
PATIENT STATUS |
|
|
In-patient |
449 (28.31%) |
|
Out-patient |
1020 (64.31%) |
The data presented below reveals that Penicillins were the most frequently prescribed class of antibiotics (38.19%) followed by Nitroimidazoles (21.43%), then Cephalosporins (15.08%). Conversely, Carbapenems and Oxazolidiones were the least prescribed classes of antibiotics (0.43% apiece), followed by the sulfonamides (0.48%), while the monobactams were never prescribed within the time frame of reference. Additional details on the antibiotics class prescribed within the specified period are contained in Table 2 below.The data presented below reveals that Penicillins were the most frequently prescribed class of antibiotics (38.19%) followed by Nitroimidazoles (21.43%), then Cephalosporins (15.08%). Conversely, Carbapenems and Oxazolidiones were the least prescribed classes of antibiotics (0.43% apiece), followed by the sulfonamides (0.48%), while the monobactams were never prescribed within the time frame of reference. Additional details on the antibiotics class prescribed within the specified period are contained in Table 2 below.
Table 2: Distribution of classes of Antibiotics Prescribed
|
ANTIBIOTICS CLASSES |
FREQUENCY (%) |
|
Penicillins |
884 (38.19%) |
|
Cephalosporins |
349 (15.08%) |
|
Sulfonamides |
11 (0.48%) |
|
Macrolides |
62 (2.68%) |
|
Quinolones/Fluoroquinolones |
196 (8.47%) |
|
Tetracyclines |
25 (1.08%) |
|
Aminoglycosides |
272 (11.75%) |
|
Carbapenems |
10 (0.43%) |
|
Monobactam |
0 (0.00%) |
|
Nitroimidazole |
496 (21.43%) |
|
Oxazolidindiones |
10 (0.43%) |
Figure 4.1: Distribution of prescription by generics
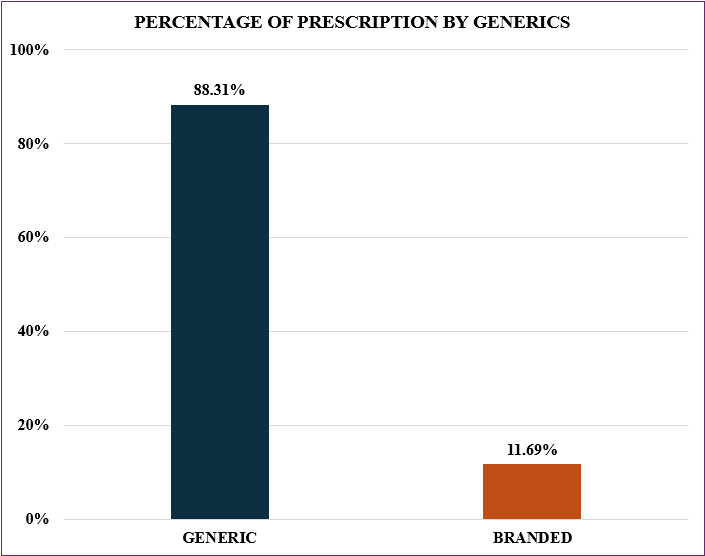
Figure 1 above indicates that the majority of prescribed antibiotics (88.31%) were generic, as opposed to 11.69% that were branded.
Figure 4.2: Distribution of antibiotics prescription by route of administration
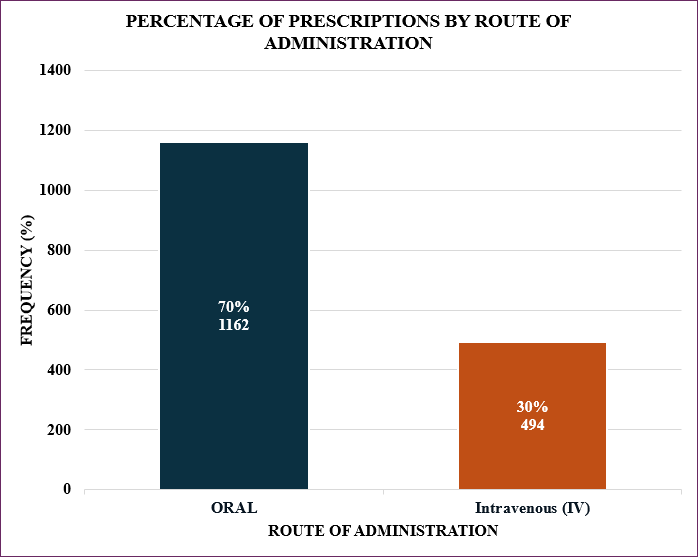
Figure 2 above indicates that the majority of the prescriptions (70%) were oral compared to the intravenous route 30%.
Discussion
The findings of this study provide valuable insights into the patterns of antibacterial agent use in a private hospital in Yenagoa, Bayelsa State, Nigeria. The demographic data reveal that the majority of prescriptions were issued to female patients (52.59%), with a significant proportion falling within the age range of 26-30 years (13.18%). Additionally, a substantial proportion of the prescriptions were for outpatients (64.31%), highlighting the importance of addressing antibiotic use in both inpatient and outpatient settings.
One of the key findings is the predominance of penicillins as the most frequently prescribed class of antibiotics (38.19%), followed by nitroimidazoles (21.43%) and cephalosporins (15.08%). This pattern aligns with previous studies that have reported a high prevalence of penicillin and cephalosporin prescriptions in healthcare settings [20,14]. However, the elevated use of nitroimidazoles, particularly metronidazole, warrants further investigation as these agents are primarily indicated for anaerobic bacterial infections and protozoal infestations [8]. Notably, the study found that the majority of prescribed antibiotics (88.31%) were generic formulations, which is consistent with efforts to promote cost-effective and accessible antimicrobial therapy [26]. This finding underscores the importance of ensuring the quality and efficacy of generic antibiotics, as well as addressing potential concerns related to substandard or counterfeit medications [15].
Regarding the route of administration, the data indicate a predominance of oral antibiotics (70%), while intravenous (IV) antibiotics accounted for 30% of prescriptions. This pattern aligns with the higher proportion of outpatient prescriptions observed in the study. However, it is essential to consider the appropriateness of the route of administration based on the clinical condition, severity of infection, and patient factors [35]. The inferential statistics revealed a significant association between age and patient status with the route of administration of prescribed antibiotics (p < 0.000). This finding suggests that factors such as age and patient status (inpatient or outpatient) may influence the choice of antibiotic route, potentially due to variations in clinical presentations, comorbidities, or treatment settings. Additionally, a significant association was observed between age and the prescription of antibiotics by generics (p < 0.000), indicating that age may play a role in determining whether generic or branded antibiotics are prescribed. These results underscore the importance of considering patient-specific factors, such as age and clinical status when prescribing antibiotics and highlight the need for tailored approaches to optimize antibiotic use across different patient populations.
The results highlight the need for targeted interventions to optimize antibiotic prescribing practices in this private hospital setting. Implementing an effective antibiotic stewardship program (ASP) could help address potential issues such as overuse, misuse, or inappropriate selection of antibiotics [2]. ASPs have been shown to improve patient outcomes, reduce healthcare costs, and minimize the risk of antimicrobial resistance [29]. Furthermore, continuous education and training for healthcare providers are crucial to ensure adherence to evidence-based guidelines, improve diagnostic accuracy, and promote judicious antibiotic prescribing [6]. Collaboration with other healthcare facilities, professional organizations, and regulatory bodies can facilitate knowledge sharing, standardization of practices, and the adoption of best practices in antibiotic stewardship [24].
Limitations
It is important to note that the interpretation of these findings should be contextualized within the limitations of the study design and setting. As a retrospective study, the data may be subject to potential biases or incomplete record-keeping. Additionally, the findings may not be generalizable to other healthcare settings or regions due to variations in prescribing practices, patient demographics, and healthcare system factors.
Conclusion
In conclusion, this study provides valuable insights into the patterns of antibacterial agent use in a private hospital in Yenagoa, Bayelsa State, Nigeria. The findings highlight the predominance of penicillins and nitroimidazoles, the preference for generic formulations, and the higher prevalence of oral antibiotics. These results underscore the need for targeted interventions, such as the implementation of antibiotic stewardship programs, continuous education, and collaboration among stakeholders, to optimize antibiotic prescribing practices, improve patient outcomes, and contribute to the global effort to combat antimicrobial resistance.
Contribution to literature: This study's findings have contributed to an existing body of knowledge that cough and other minor reasons are implicated in antibiotic use in this part of the world.
Acknowledgment: The researchers appreciate the statistician, respondents, and co-researchers for the time.
Conflict of Interest: The researchers declare that there was no conflict of interest.
- Aldred, K. J, Kerns, R. J, Osheroff, N. (2014). Mechanism of quinolone action and resistance. Biochemistry, 53(10), 1565-1574.
- Barlam, T. F, Cosgrove, S. E, Abbo, L. M, MacDougall, C, Schuetz, A. N, Septimus, E. J, Trivedi, K. K. (2016). Implementing an antibiotic stewardship program: guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clinical Infectious Diseases, 62(10), 51-77.
- Bushby, S. R. M, Hitchings, G. H. (1968). Trimethoprim, a sulphonamide potentiator. British Journal of Pharmacology and Chemotherapy, 33(1), 72–90.
- Centers for Disease Control and Prevention. (2019). Antibiotic resistance threats in the United States,
- Chopra, I, Roberts, M. (2001). Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiology and Molecular Biology Reviews, 65(2), 232–260.
- Courtenay, M, Rowbotham, S, Lim, R, Peters, S, Yates, K, Chater, A. (2018). Examining what matters: Framing quality improvement efforts for better understanding of success and impact. BMC Health Services Research, 18(1), 1–12.
- Cunha, B. A. (2019). Aminoglycoside therapy of gram-negative bacillary infections. The Antibiotics Manual: A Guide to Commonly Used Antimicrobials, 2nd ed, updated. World Scientific.
- Cushnie, T. P. T, Cushnie, B, Lamb, A. J. (2003). Detection of mutagenic, antimicrobial, and antioxidant activities in lichens--a new source of bioactive compounds. Biochemical Society Transactions, 31(6), 1384–1389.
- Dik, J. W, Poelman, R, Friedrich, A. W, Niesters, H. G, Rossen, J. W, Senne, M. (2016). An integrated, heterogeneous, next-generation sequencing-based diagnostic workflow for detecting and quantifying bacterial pathogens in the clinical setting. BMC Microbiology, 16(1), 1-10.
- Dyar, O. J, Tulu, N, Griffin, M. R. (2014). Patterns of antimicrobial use and antimicrobial resistance among healthcare facility-associated pathogens in Burlington, Vermont, 2012–2014. American Journal of Infection Control, 42(3), 296–301.
- Gandra, S, Barter, D. M, Laxminarayan, R. (2014). Economic burden of antibiotic resistance: how much do we know? Clinical Microbiology and Infection, 20(10), 973-980.
- Gualano, M. R, Gili, R, Scaioli, G, Bert, F, Siliquini, R. (2015). General population's knowledge and attitudes about antibiotics: a systematic review and meta-analysis. Pharmacoepidemiology and Drug Safety, 24(1), 2-10.
- Hutchings, M. I, Truman, A. W, Wilkinson, B. (2019). Antibiotics: past, present and future. Current Opinion in Microbiology, 51, 72-80.
- Isah, A. O, Ross-Degnan, D, Quick, J, Laing, R, Mabadeje, A. F. B. (2002). The development of standard values for the WHO drug use prescribing indicators. International Conference on Improving Use of Medicines, Chiang Mai, Thailand.
- Jasovsky, D, Gerdes, H, Underwood, K, Laxminarayan, R. (2020). An economic framework for preventive and mitigative interventions against antimicrobial resistance. PLoS ONE, 15(10), 0240519.
- Jasovský, D, Littmann, J, Zorzet, A, Cars, O. (2016). Antimicrobial resistance-a threat to the world's sustainable development. Upsala journal of medical sciences, 121(3), 159–164.
- Kahne, D, Leimkuhler, C, Lu, W, Walsh, C. (2005). Glycopeptide and lipoglycopeptide antibiotics. Chemical Reviews, 105(2), 425-448.
- Kapoor, G, Saigal, S, Elongavan, A. (2017). Action and resistance mechanisms of antibiotics: A guide for clinicians. Journal of Anaesthesiology, Clinical Pharmacology, 33(3), 300–305.
- Katzung, B. G, & Trevor, A. J. (2015). Basic & clinical pharmacology (13th ed.). McGraw-Hill Education.
- Klein, E. Y, Van Boeckel, T. P, Martinez, E. M, Pant, S, Gandra, S, Leung, S. A, Laxminarayan, R. (2018). Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proceedings of the National Academy of Sciences, 115(15), 3463-3470.
- Kohanski, M. A, Dwyer, D. J, Collins, J. J. (2010). How antibiotics kill bacteria: from targets to networks. Nature Reviews Microbiology, 8(6), 423–435.
- Kraemer, S. A, Ramachandran, A, Perron, G. G. (2019). Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms, 7(6), 180.
- McCullough, A. R, Parekh, S, Rathbone, J, Del Mar, C. B, Hoffmann, T. C. (2016). A systematic review of the public's knowledge and beliefs about antibiotic resistance. Journal of Antimicrobial Chemotherapy, 71(1), 27–33.
- Mendelson, M, Matsoso, M. P. (2015). The World Health Organization Global Action Plan for Antimicrobial Resistance. South African Medical Journal, 105(5), 325-325.
- Morel, C. M, Lindahl, O, Harbarth, S, de Velde, S. V, Holmström, P, Gorris, M. E. (2017). Understanding doctors' attitudes towards antibiotic use and antimicrobial resistance in the Netherlands. Journal of Antimicrobial Chemotherapy, 72(6), 1719-1727.
- Okeke, I. N, Lamikanra, A, Edelman, R. (1999). Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerging Infectious Diseases, 5(1), 18-27.
- Shapiro, D. J, Hicks, L. A, Pavia, A. T, Hersh, A. L. (2014). Antibiotic prescribing for adults in ambulatory care in the USA, 2007–09. Journal of Antimicrobial Chemotherapy, 69(1), 234–240.
- Shrestha, P, Cooper, B. S, Coast, J, Oppong, R, Do Thi Thuy, N, Phodha, T, Lubell, Y. (2018). Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrobial Resistance & Infection Control, 7(1), 1–10.
- Srinivasan, A, Song, X, Perl, T. M, Ackerman, G, Nordan, J, McGee, L. (2016). Antimicrobial stewardship and measures of resource utilization in academic hospitals in southwestern Pennsylvania in 2006 and 2010. Antimicrobial Resistance & Infection Control, 5(1), 1–8.
- Tamma, P. D, Avdic, E, Li, D. X, Dzintars, K, Cosgrove, S. E. (2017). Association of adverse events with antibiotic use in hospitalized patients. JAMA Internal Medicine, 177(9), 1308-1315.
- Teillant, A, Gandra, S, Barter, D, Morgan, D. J, Laxminarayan, R. (2015). Potential burden of antibiotic resistance on surgery and cancer chemotherapy antibiotic prophylaxis in the USA: a literature review and modeling study. The Lancet Infectious Diseases, 15(12), 1429-1437.
- Tonkin-Crine, S, Yardley, L, Little, P. (2011). Antibiotic prescribing for acute respiratory tract infections in primary care: a systematic review and meta-ethnography. Journal of Antimicrobial Chemotherapy, 66(10), 2215–2223.
- Trimble, M. J, Mlynárčik, P, Kolář, M, Hancock, R. E. W. (2016). Polymyxin: Alternative mechanisms of action and resistance. Cold Spring Harbor Perspectives in Medicine, 6(10), 025288.
- Van Boeckel, T. P, Brower, C, Gilbert, M, Grenfell, B. T, Levin, S. A, Robinson, T. P, Laxminarayan, R. (2015). Global trends in antimicrobial use in food animals. Proceedings of the National Academy of Sciences, 112(18), 5649–5654.
- Ventola, C. L. (2015). The antibiotic resistance crisis: Part 1: causes and threats. P & T: A peer-reviewed journal for formulary management, 40(4), 277-283.
- Wilke, M. S, Lovering, A. L, Strynadka, N. C. J. (2005). β-Lactam antibiotic resistance: A current structural perspective. Current Opinion in Microbiology, 8(5), 525-533.
- World Health Organization. (2016). Guidelines on core components of infection prevention and control programs at the national and acute health care facility level.
- World Health Organization. (2020). Antimicrobial resistance.
Download Provisional PDF Here
PDF
